| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation date | 2015-10-09 22:29:18 UTC |
|---|
| Update date | 2017-01-19 02:36:17 UTC |
|---|
| FoodComEx ID | PC000223 |
|---|
| FoodDB Record | FDB022694 |
|---|
| Chemical Information |
|---|
| Name | Epi-coprostanol |
|---|
| Description | Epi-coprostanol is a 27 carbon stanol formed from the biohydrogenation of cholesterol (cholest-5en-3β-ol) in the gut of most higher animals and birds. It is a breakdown product of 5b-coprastanol and can be found in treated sewage. It is considered to be an antioxidant and is a major constituent of ambergris. [HMDB] |
|---|
| CAS Number | 516-95-0 |
|---|
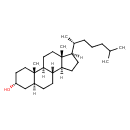
| Structure | |
|---|
| Synonyms | | Synonym | Source |
|---|
| (3-a,5-a)-Cholestan-3-ol | Generator | | (3-alpha,5-alpha)-Cholestan-3-ol | ChEBI | | (3-α,5-α)-cholestan-3-ol | Generator | | 3a-Hydroxy-5a-cholestane | Generator | | 3alpha-Hydroxy-5alpha-cholestane | ChEBI | | 3α-hydroxy-5α-cholestane | Generator | | 5a-Cholestan-3a-ol | Generator | | 5alpha-Cholestan-3alpha-ol | ChEBI | | 5b-Cholestan-3a-ol | hmdb | | 5b-Cholestane-3a-ol | hmdb | | 5b-Cholestanol | hmdb | | 5beta-Cholestan-3alpha-ol | hmdb | | 5beta-Cholestane-3alpha-ol | hmdb | | 5beta-Cholestanol | hmdb | | 5α-cholestan-3α-ol | Generator | | a-Coprostanol | hmdb | | alpha-Coprostanol | hmdb | | Epi-cholestanol | ChEBI | | Epi-coprostanol | hmdb | | Epi-coprosterol | hmdb | | Epicoprostanol | hmdb | | Epicoprosterol | hmdb | | Epidehydrocholesterin | ChEBI | | Epidihydrocholesterin | ChEBI | | Presteron | ChEBI |
|
|---|
| Chemical Formula | C27H48O |
|---|
| IUPAC name | (1S,2S,5R,7S,10R,11S,14R,15R)-2,15-dimethyl-14-[(2R)-6-methylheptan-2-yl]tetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadecan-5-ol |
|---|
| InChI Identifier | InChI=1S/C27H48O/c1-18(2)7-6-8-19(3)23-11-12-24-22-10-9-20-17-21(28)13-15-26(20,4)25(22)14-16-27(23,24)5/h18-25,28H,6-17H2,1-5H3/t19-,20+,21-,22+,23-,24+,25+,26+,27-/m1/s1 |
|---|
| InChI Key | QYIXCDOBOSTCEI-FBVYSKEZSA-N |
|---|
| Isomeric SMILES | [H][C@@]1(CC[C@@]2([H])[C@]3([H])CC[C@@]4([H])C[C@H](O)CC[C@]4(C)[C@@]3([H])CC[C@]12C)[C@H](C)CCCC(C)C |
|---|
| Average Molecular Weight | 388.6694 |
|---|
| Monoisotopic Molecular Weight | 388.370516158 |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as cholesterols and derivatives. Cholesterols and derivatives are compounds containing a 3-hydroxylated cholestane core. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Steroids and steroid derivatives |
|---|
| Sub Class | Cholestane steroids |
|---|
| Direct Parent | Cholesterols and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Cholesterol-skeleton
- Cholesterol
- 3-alpha-hydroxysteroid
- Hydroxysteroid
- 3-hydroxysteroid
- Cyclic alcohol
- Secondary alcohol
- Organic oxygen compound
- Hydrocarbon derivative
- Organooxygen compound
- Alcohol
- Aliphatic homopolycyclic compound
|
|---|
| Molecular Framework | Aliphatic homopolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Physico-Chemical Properties - Experimental |
|---|
| Property | Value | Reference |
|---|
| Experimental logP | Not Available | |
|---|
| Experimental Water Solubility | Not Available | |
|---|
| Melting Point | Not Available | |
|---|
|
| Foods of Origin |
|---|
| Food | Content Range | Average | Reference |
|---|
| Food | | | Reference |
|---|
|
| Production Data |
|---|
| Production Method | commercial |
|---|
| Production Method Reference | Not Available |
|---|
| Production Method Reference File | Not Available |
|---|
| Quantity Available | Production upon request, up to 400 mg |
|---|
| Delivery Time | Not Available |
|---|
| Storage Form | solid |
|---|
| Storage Conditions | -80°C |
|---|
| Stability | Not Available |
|---|
| Purity | Not Available |
|---|
| Spectra |
|---|
| Spectral Data Upon Request | Not Available |
|---|
| Provider Information |
|---|
|
| Commercial Vendors |
|---|
| Not Available |