| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation date | 2015-10-09 22:29:17 UTC |
|---|
| Update date | 2017-01-19 02:36:17 UTC |
|---|
| FoodComEx ID | PC000221 |
|---|
| FoodDB Record | FDB022254 |
|---|
| Chemical Information |
|---|
| Name | Pyrrolidonecarboxylic acid |
|---|
| Description | 2-Pyrrolidone-5-carboxylic acid (PCA) is a cyclic derivative of glutamic acid, physiologically present in mammalian tissues. It has been shown that PCA releases GABA from the cerebral cortex and displays anti-anxiety effects in a simple approach-avoidance conflict situation in the rat. In clinical pharmacology experiments, PCA significantly shortens the plasma half-life of ethanol during acute intoxication. [HMDB] |
|---|
| CAS Number | 4042-36-8 |
|---|
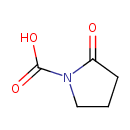
| Structure | |
|---|
| Synonyms | | Synonym | Source |
|---|
| (+)-2-Pyrrolidone-5-carboxylate | hmdb | | (+)-2-Pyrrolidone-5-carboxylic acid | hmdb | | (+)-Pyroglutamate | hmdb | | (+)-Pyroglutamic acid | hmdb | | (2R)-2-Carboxy-5-pyrrolidinone | hmdb | | (R)-(+)-2-Pyrrolidone-5-carboxylate | hmdb | | (R)-(+)-2-Pyrrolidone-5-carboxylic acid | hmdb | | (R)-2-Pyrrolidone-5-carboxylate | hmdb | | (R)-2-Pyrrolidone-5-carboxylic acid | hmdb | | (R)-5-Oxopyrrolidine-2-carboxylate | hmdb | | (R)-5-Oxopyrrolidine-2-carboxylic acid | hmdb | | 2-Pyrrolidone-5-carboxylate | Generator | | 2-Pyrrolidone-5-carboxylic acid | ChEBI | | 5-Oxo-D-proline | hmdb | | 5-oxo-DL-Proline | ChEBI | | D-2-Pyrrolidone-5-carboxylic | hmdb | | D-5-Pyrrolidone-2-carboxylate | hmdb | | D-5-Pyrrolidone-2-carboxylic acid | hmdb | | D-Pyroglutamate | hmdb | | D-Pyroglutamic acid | hmdb | | Glp | ChEBI |
|
|---|
| Chemical Formula | C5H7NO3 |
|---|
| IUPAC name | 2-oxopyrrolidine-1-carboxylic acid |
|---|
| InChI Identifier | InChI=1S/C5H7NO3/c7-4-2-1-3-6(4)5(8)9/h1-3H2,(H,8,9) |
|---|
| InChI Key | DQAKJEWZWDQURW-UHFFFAOYSA-N |
|---|
| Isomeric SMILES | OC(=O)N1CCCC1=O |
|---|
| Average Molecular Weight | 129.114 |
|---|
| Monoisotopic Molecular Weight | 129.042593095 |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as oxoprolines. Oxoprolines are compounds containing an oxoproline moiety, which consists of a pyrrolidine ring bearing a carboxylic acid group at the ring position 2, and a ketone group. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Pyrrolidines |
|---|
| Sub Class | Pyrrolidones |
|---|
| Direct Parent | Oxoprolines |
|---|
| Alternative Parents | |
|---|
| Substituents | - Oxoproline
- Pyrrolidine carboxylic acid
- Pyrrolidine carboxylic acid or derivatives
- 2-pyrrolidone
- Dicarboximide
- Carbonic acid derivative
- Lactam
- Carbamic acid derivative
- Carbamic acid
- Azacycle
- Carboxylic acid derivative
- Organic oxide
- Organopnictogen compound
- Organic oxygen compound
- Organic nitrogen compound
- Organooxygen compound
- Organonitrogen compound
- Carbonyl group
- Hydrocarbon derivative
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Physico-Chemical Properties - Experimental |
|---|
| Property | Value | Reference |
|---|
| Experimental logP | Not Available | |
|---|
| Experimental Water Solubility | Not Available | |
|---|
| Melting Point | Not Available | |
|---|
|
| Foods of Origin |
|---|
| Food | Content Range | Average | Reference |
|---|
| Food | | | Reference |
|---|
|
| Production Data |
|---|
| Production Method | commercial |
|---|
| Production Method Reference | Not Available |
|---|
| Production Method Reference File | Not Available |
|---|
| Quantity Available | Production upon request, up to 3 g |
|---|
| Delivery Time | Not Available |
|---|
| Storage Form | solid |
|---|
| Storage Conditions | -80°C |
|---|
| Stability | Not Available |
|---|
| Purity | Not Available |
|---|
| Spectra |
|---|
| Spectral Data Upon Request | Not Available |
|---|
| Provider Information |
|---|
|
| Commercial Vendors |
|---|
| Not Available |