| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation date | 2015-10-09 22:29:12 UTC |
|---|
| Update date | 2017-01-19 02:36:16 UTC |
|---|
| FoodComEx ID | PC000208 |
|---|
| FoodDB Record | FDB021818 |
|---|
| Chemical Information |
|---|
| Name | Glucose 6-phosphate |
|---|
| Description | Glucose 6 phosphate (alpha-D-glucose 6 phosphate or G6P) is the alpha-anomer of glucose-6-phosphate. There are two anomers of glucose 6 phosphate, the alpha anomer and the beta anomer. Glucose 6 phosphate is an ester of glucose with phosphoric acid, made in the course of glucose metabolism by mammalian and other cells. It is a normal constituent of resting muscle and probably is in constant equilibrium with fructose-6-phosphate. (Stedman, 26th ed). Glucose-6-phosphate is a phosphorylated glucose molecule on carbon 6. When glucose enters a cell, it is immediately phosphorylated to G6P. This is catalyzed with hexokinase enzymes, thus consuming one ATP. A major reason for immediate phosphorylation of the glucose is so that it cannot diffuse out of the cell. The phosphorylation adds a charged group so the G6P cannot easily cross cell membranes. G6P can travel down two metabolic pathways, glycolysis and the pentose phosphate pathway. In addition to the metabolic pathways, G6P can also be stored as glycogen in the liver if blood glucose levels are high. If the body needs energy or carbon skeletons for syntheses, G6P can be isomerized to Fructose-6-phosphate and then phosphorylated to Fructose-1,6-bisphosphate. Note, the molecule now has 2 phosphoryl groups attached. The addition of the 2nd phosphoryl group is an irreversible step, so once this happens G6P will enter glycolysis and be turned into pyruvate (ATP production occurs). If blood glucose levels are high, the body needs a way to store the excess glucose. After being converted to G6P, phosphoglucose mutase (isomerase) can turn the molecule into glucose-1-phosphate. Glucose-1-phosphate can then be combined with uridine triphosphate (UTP) to form UDP-glucose. This reaction is driven by the hydrolysis of pyrophosphate that is released in the reaction. Now, the activated UDP-glucose can add to a growing glycogen molecule with the help of glycogen synthase. This is a very efficient storage mechanism for glucose since it costs the body only 1 ATP to store the 1 glucose molecule and virtually no energy to remove it from storage. It is important to note that glucose-6-phosphate is an allosteric activator of glycogen synthase, which makes sense because when the level of glucose is high the body should store the excess glucose as glycogen. On the other hand, glycogen synthase is inhibited when it is phosphorylated by protein kinase a during times of high stress or low blood glucose levels. -- Wikipedia [HMDB] |
|---|
| CAS Number | 56-73-5 |
|---|
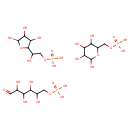
| Structure | |
|---|
| Synonyms | | Synonym | Source |
|---|
| 6-O-ONO-D-Glucopyranose | ChEBI | | a-D-Glucose 6- ate | HMDB | | alpha-D-Glucose 6- ate | HMDB | | alpha-D-Glucose 6-ate | HMDB | | alpha-D-Hexose 6-ate | HMDB | | D-Glucose 6-ate | ChEBI | | D-Glucose 6-ic acid | Generator | | D-Glucose-6-dihydrogen ate | HMDB | | D-Hexose 6-ate | HMDB | | D(+)-Glucopyranose 6-ate | HMDB | | Glc6P | ChEBI | | Glucose 6-ate | HMDB | | Glucose-6-ate | HMDB | | Robison ester | ChEBI |
|
|---|
| Chemical Formula | C18H39O27P3 |
|---|
| IUPAC name | [(2,3,4,5-tetrahydroxy-6-oxohexyl)oxy]phosphonic acid; [(3,4,5,6-tetrahydroxyoxan-2-yl)methoxy]phosphonic acid; [2-hydroxy-2-(3,4,5-trihydroxyoxolan-2-yl)ethoxy]phosphonic acid |
|---|
| InChI Identifier | InChI=1S/3C6H13O9P/c7-2(1-14-16(11,12)13)5-3(8)4(9)6(10)15-5;7-3-2(1-14-16(11,12)13)15-6(10)5(9)4(3)8;7-1-3(8)5(10)6(11)4(9)2-15-16(12,13)14/h2*2-10H,1H2,(H2,11,12,13);1,3-6,8-11H,2H2,(H2,12,13,14) |
|---|
| InChI Key | NVBMVWKTLWOTGR-UHFFFAOYSA-N |
|---|
| Isomeric SMILES | OC(COP(O)(O)=O)C(O)C(O)C(O)C=O.OC(COP(O)(O)=O)C1OC(O)C(O)C1O.OC1OC(COP(O)(O)=O)C(O)C(O)C1O |
|---|
| Average Molecular Weight | 780.4073 |
|---|
| Monoisotopic Molecular Weight | 780.089155578 |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as hexose phosphates. These are carbohydrate derivatives containing a hexose substituted by one or more phosphate groups. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic oxygen compounds |
|---|
| Class | Organooxygen compounds |
|---|
| Sub Class | Carbohydrates and carbohydrate conjugates |
|---|
| Direct Parent | Hexose phosphates |
|---|
| Alternative Parents | |
|---|
| Substituents | - Hexose phosphate
- Monosaccharide phosphate
- Monoalkyl phosphate
- Alkyl phosphate
- Phosphoric acid ester
- Oxane
- Organic phosphoric acid derivative
- Tetrahydrofuran
- Secondary alcohol
- Hemiacetal
- Organoheterocyclic compound
- Polyol
- Oxacycle
- Alcohol
- Hydrocarbon derivative
- Organic oxide
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Not Available |
|---|
| External Descriptors | Not Available |
|---|
| Physico-Chemical Properties - Experimental |
|---|
| Property | Value | Reference |
|---|
| Experimental logP | Not Available | |
|---|
| Experimental Water Solubility | Not Available | |
|---|
| Melting Point | Not Available | |
|---|
|
| Foods of Origin |
|---|
| Food | Content Range | Average | Reference |
|---|
| Food | | | Reference |
|---|
|
| Production Data |
|---|
| Production Method | commercial |
|---|
| Production Method Reference | Not Available |
|---|
| Production Method Reference File | Not Available |
|---|
| Quantity Available | Production upon request, up to 2 g |
|---|
| Delivery Time | Not Available |
|---|
| Storage Form | solid |
|---|
| Storage Conditions | -80°C |
|---|
| Stability | Not Available |
|---|
| Purity | Not Available |
|---|
| Spectra |
|---|
| Spectral Data Upon Request | Not Available |
|---|
| Provider Information |
|---|
|
| Commercial Vendors |
|---|
| Toronto Research Chemicals | G595338 |
|---|