| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation date | 2015-10-09 22:29:06 UTC |
|---|
| Update date | 2017-01-19 02:36:15 UTC |
|---|
| FoodComEx ID | PC000184 |
|---|
| FoodDB Record | FDB012596 |
|---|
| Chemical Information |
|---|
| Name | Histamine |
|---|
| Description | An amine derived by enzymatic decarboxylation of histidine. It is a powerful stimulant of gastric secretion, a constrictor of bronchial smooth muscle, a vasodilator, and also a centrally acting neurotransmitter.; Histamine is a biogenic amine involved in local immune responses as well as regulating physiological function in the gut and acting as a neurotransmitter. Histamine triggers the inflammatory response. As part of an immune response to foreign pathogens, histamine is produced by basophils and by mast cells found in nearby connective tissues. Histamine increases the permeability of the capillaries to white blood cells and other proteins, in order to allow them to engage foreign invaders in the affected tissues. It is found in virtually all animal body cells.[citation needed]; Histamine is derived from the decarboxylation of the amino acid histidine, a reaction catalyzed by the enzyme L-histidine decarboxylase. It is a hydrophilic vasoactive amine. |
|---|
| CAS Number | 51-45-6 |
|---|
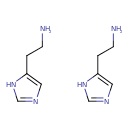
| Structure | |
|---|
| Synonyms | | Synonym | Source |
|---|
| β-aminoethylglyoxaline | biospider | | β-aminoethylimidazole | biospider | | β-Imidazolyl-4-ethylamine | biospider | | 1H-Imidazole-4-ethanamine | ChEBI | | 1H-Imidazole-4-ethanamine, 9CI | db_source | | 2-(4-Imidazolyl)ethylamine | ChEBI | | 4-(2-Aminoethyl)-1H-imidazole | db_source | | Ergamine | db_source | | Histaminum | biospider | | Histaminum hydrochloricum | biospider | | Histaminum muriaticum | biospider | | Scombrotoxin | db_source |
|
|---|
| Chemical Formula | C10H18N6 |
|---|
| IUPAC name | bis(2-(1H-imidazol-5-yl)ethan-1-amine) |
|---|
| InChI Identifier | InChI=1S/2C5H9N3/c2*6-2-1-5-3-7-4-8-5/h2*3-4H,1-2,6H2,(H,7,8) |
|---|
| InChI Key | CLIIFKDZHSLNHY-UHFFFAOYSA-N |
|---|
| Isomeric SMILES | NCCC1=CN=CN1.NCCC1=CNC=N1 |
|---|
| Average Molecular Weight | 222.2901 |
|---|
| Monoisotopic Molecular Weight | 222.159294606 |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as 2-arylethylamines. These are primary amines that have the general formula RCCNH2, where R is an organic group. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic nitrogen compounds |
|---|
| Class | Organonitrogen compounds |
|---|
| Sub Class | Amines |
|---|
| Direct Parent | 2-arylethylamines |
|---|
| Alternative Parents | |
|---|
| Substituents | - 2-arylethylamine
- Aralkylamine
- Heteroaromatic compound
- Imidazole
- Azole
- Azacycle
- Organoheterocyclic compound
- Organopnictogen compound
- Hydrocarbon derivative
- Primary aliphatic amine
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Not Available |
|---|
| External Descriptors | Not Available |
|---|
| Physico-Chemical Properties - Experimental |
|---|
| Property | Value | Reference |
|---|
| Experimental logP | -0.70 | SANGSTER (1993) |
|---|
| Experimental Water Solubility | Not Available | |
|---|
| Melting Point | Mp 86° | DFC |
|---|
|
| Foods of Origin |
|---|
| Food | Content Range | Average | Reference |
|---|
| Food | | | Reference |
|---|
|
| Production Data |
|---|
| Production Method | commercial |
|---|
| Production Method Reference | Not Available |
|---|
| Production Method Reference File | Not Available |
|---|
| Quantity Available | Production upon request, up to 1 g |
|---|
| Delivery Time | Not Available |
|---|
| Storage Form | solid |
|---|
| Storage Conditions | -80°C |
|---|
| Stability | Not Available |
|---|
| Purity | Not Available |
|---|
| Spectra |
|---|
| Spectral Data Upon Request | Not Available |
|---|
| Provider Information |
|---|
|
| Commercial Vendors |
|---|
| MetaSci | HMDB0000870 |
|---|
| Sigma-Aldrich | HMDB0000870 |
|---|
| Toronto Research Chemicals | H436503 |
|---|