| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation date | 2015-10-09 22:29:03 UTC |
|---|
| Update date | 2017-01-19 02:36:15 UTC |
|---|
| FoodComEx ID | PC000176 |
|---|
| FoodDB Record | FDB012530 |
|---|
| Chemical Information |
|---|
| Name | D-Glucose |
|---|
| Description | Glucose is a monosaccharide containing six carbon atoms and an aldehyde group. It is referred to as an aldohexose. The glucose molecule can exist in an open-chain (acyclic) and ring (cyclic) form, the latter being the result of an intramolecular reaction between the aldehyde C atom and the C-5 hydroxyl group to form an intramolecular hemiacetal. In aqueous solution, both forms are in equilibrium and at pH 7 the cyclic one is predominant. Glucose is a primary source of energy for all living organisms. It is a fundamental metabolite found in all organisms, ranging from bacteria to plants to humans. Most of the glucose generated on the Earth is made by plants and algae during photosynthesis from water and carbon dioxide, where it is used to make cellulose (and other polymeric forms of glucose called polysaccharides) that stabilize plant cell walls. Glucose is also found in fruits and other parts of plants in its free state. In animals, glucose can be generated from the breakdown of glycogen in a process known as glycogenolysis. Glucose can also be synthesized de novo in animals. In particular, it can be synthesized in the liver and kidneys from non-carbohydrate intermediates, such as pyruvate and glycerol (and gluconeogenic amino acids such as glycine, serine and alanine), by a process known as gluconeogenesis. Humans also consume large amounts of glucose as part of their regular diet. Ingested glucose initially binds to the receptor for sweet taste on the tongue in humans. This complex of the proteins T1R2 and T1R3 makes it possible to identify glucose-containing food sources. Glucose in the body mainly comes from food - about 300 g per day for the average adult. In humans, the breakdown of glucose-containing polysaccharides happens partly during chewing by means of the enzyme known as amylase, which is contained in saliva, as well as by other enzymes such as maltase, lactase and sucrase on the brush border of the small intestine. The blood sugar content of a healthy person in the short-time fasting state, e.g. after overnight fasting, is about 70 to 100 mg/dL of blood (4 to 5.5 mM). In blood plasma, the measured values are about 10-15% higher. Dysregulated metabolism of glucose can lead to a number of diseases including diabetes. Diabetes is a metabolic disorder where the body is unable to regulate levels of glucose in the blood either because of a lack of insulin in the body or the failure, by cells in the body, to respond properly to insulin. Each of these situations can be caused by persistently high elevations of blood glucose levels (called hyperglycemia), through pancreatic burnout and insulin resistance. Persistently elevated levels of glucose (>6 mM or >120 mg/dL) can lead to the formation of covalent adducts of glucose with plasma proteins through a non-enzymatic process known as glycation. This glycation reaction leads to advanced glycation end products or AGEs (PMID: 24634591 ). AGEs are thought to be the major causes of different diabetic complications. High glucose levels may induce glycation of various structural and functional proteins including plasma proteins and collagen. The non-enzymatic modification of plasma proteins such as albumin, fibrinogen, hemoglobin and globulins may produce various deleterious effects including alteration in drug binding in the plasma, platelet activation, generation of oxygen free radicals, impaired fibrinolysis and impairment in immune system regulation (PMID: 24634591 ). Transiently elevated glucose (up to 7.3 mM or 133 mg/dL) is often seen shortly after the consumption of a meal or a food item that is rich in carbohydrates -- even among very healthy people (PMID: 19885137 ). Glucose is also elevated when an individual is fighting viral or bacterial infections or suffering from traumatic injuries (burns, wounds). In fact, glucose can be significantly elevated (>11 mM or 200 mg/dL) when individuals are experiencing sepsis or septic shock (PMID: 16006275 ). On the other hand, low blood glucose levels (hypoglycemia) where blood glucose is <3.9 mM (70 mg/dL) are common among people with type 1 diabetes and people with type 2 diabetes who take certain diabetic medicines. Certain conditions, such as liver disease, may also cause low levels of blood glucose. Hypoglycemia can lead to fatigue, sleepiness, short temper or feeling faint. |
|---|
| CAS Number | 492-61-5 |
|---|
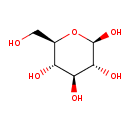
| Structure | |
|---|
| Synonyms | | Synonym | Source |
|---|
| (+)-glucose | biospider | | Anhydrous dextrose | HMDB | | Cerelose | HMDB | | Cerelose 2001 | HMDB | | Clearsweet 95 | HMDB | | Clintose L | HMDB | | Corn sugar | HMDB | | CPC Hydrate | HMDB | | D-(+)-glucose | biospider | | D-Glc | ChEBI | | D-Glcp | ChEBI | | D(+)-glucose | biospider | | Dextropur | HMDB | | Dextrose | manual | | Dextrosol | HMDB | | Glucodin | HMDB | | Glucolin | HMDB | | Glucose | ChEBI | | Goldsugar | HMDB | | Grape sugar | manual | | Meritose | HMDB | | Roferose st | HMDB | | Staleydex 111 | HMDB | | Staleydex 95m | HMDB | | Tabfine 097(hs) | HMDB | | Vadex | HMDB |
|
|---|
| Chemical Formula | C6H12O6 |
|---|
| IUPAC name | (2R,3R,4S,5S,6R)-6-(hydroxymethyl)oxane-2,3,4,5-tetrol |
|---|
| InChI Identifier | InChI=1S/C6H12O6/c7-1-2-3(8)4(9)5(10)6(11)12-2/h2-11H,1H2/t2-,3-,4+,5-,6-/m1/s1 |
|---|
| InChI Key | WQZGKKKJIJFFOK-VFUOTHLCSA-N |
|---|
| Isomeric SMILES | OC[C@H]1O[C@@H](O)[C@H](O)[C@@H](O)[C@@H]1O |
|---|
| Average Molecular Weight | 180.1559 |
|---|
| Monoisotopic Molecular Weight | 180.063388116 |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as hexoses. These are monosaccharides in which the sugar unit is a is a six-carbon containing moeity. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic oxygen compounds |
|---|
| Class | Organooxygen compounds |
|---|
| Sub Class | Carbohydrates and carbohydrate conjugates |
|---|
| Direct Parent | Hexoses |
|---|
| Alternative Parents | |
|---|
| Substituents | - Hexose monosaccharide
- Oxane
- Secondary alcohol
- Hemiacetal
- Oxacycle
- Organoheterocyclic compound
- Polyol
- Hydrocarbon derivative
- Primary alcohol
- Alcohol
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Physico-Chemical Properties - Experimental |
|---|
| Property | Value | Reference |
|---|
| Experimental logP | -3.24 | SANGSTER (1994) |
|---|
| Experimental Water Solubility | 1200 mg/mL at 30 oC | MULLIN,JW (1972) |
|---|
| Melting Point | 146-150 oC | |
|---|
|
| Foods of Origin |
|---|
| Food | Content Range | Average | Reference |
|---|
| Food | | | Reference |
|---|
|
| Production Data |
|---|
| Production Method | commercial |
|---|
| Production Method Reference | Not Available |
|---|
| Production Method Reference File | Not Available |
|---|
| Quantity Available | Production upon request, up to 6 g |
|---|
| Delivery Time | Not Available |
|---|
| Storage Form | solid |
|---|
| Storage Conditions | -80°C |
|---|
| Stability | Not Available |
|---|
| Purity | Not Available |
|---|
| Spectra |
|---|
| Spectral Data Upon Request | Not Available |
|---|
| Provider Information |
|---|
|
| Commercial Vendors |
|---|
| AKSci | 7531AF |
|---|
| Cayman Chemical | 16775 |
|---|