| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation date | 2015-10-09 22:29:02 UTC |
|---|
| Update date | 2017-01-19 02:36:15 UTC |
|---|
| FoodComEx ID | PC000171 |
|---|
| FoodDB Record | FDB021908 |
|---|
| Chemical Information |
|---|
| Name | NADP |
|---|
| Description | Nicotinamide adenine dinucleotide phosphate. A coenzyme composed of ribosylnicotinamide 5-phosphate (NMN) coupled by pyrophosphate linkage to the 5-phosphate adenosine 2,5-bisphosphate. It serves as an electron carrier in a number of reactions, being alternately oxidized (NADP+) and reduced (NADPH). (Dorland, 27th ed.) Hydrogen carrier in biochemical redox systems. In the hexose monophosphoric acid system it is reduced to Dihydrocoenzyme II and reoxidation in the presence of flavoproteins (Dictionary of Organic Compounds) [HMDB]. NADP is found in many foods, some of which are black walnut, lemon verbena, savoy cabbage, and silver linden. |
|---|
| CAS Number | 53-59-8 |
|---|
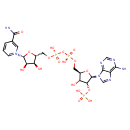
| Structure | |
|---|
| Synonyms | | Synonym | Source |
|---|
| Adenine-nicotinamide dinucleotide ate | HMDB | | Adenine-nicotinamide dinucleotide phosphate | hmdb | | b-NADP | hmdb | | b-Nicotinamide adenine dinucleotide ate | Generator | | b-Nicotinamide adenine dinucleotide ic acid | Generator | | b-Nicotinamide adenine dinucleotide phosphate | hmdb | | b-TPN | hmdb | | beta-NADP | hmdb | | beta-Nicotinamide adenine dinucleotide ate | ChEBI | | beta-Nicotinamide adenine dinucleotide ic acid | Generator | | beta-Nicotinamide adenine dinucleotide phosphate | hmdb | | beta-TPN | hmdb | | Codehydrase II | hmdb | | Codehydrogenase II | hmdb | | Coenzyme II | hmdb | | Cozymase II | hmdb | | NAD ate | HMDB | | NAD phosphate | hmdb | | NADP | hmdb | | NADP+ | hmdb | | Nicotinamide adenine dinucleotide ate | ChEBI | | Nicotinamide adenine dinucleotide ic acid | Generator | | Nicotinamide adenine dinucleotide phosphate | hmdb | | Nicotinamide-adenine dinucleotide ate | HMDB | | Nicotinamide-adenine dinucleotide phosphate | hmdb | | Oxidized nicotinamide-adenine dinucleotide ate | ChEBI | | Oxidized nicotinamide-adenine dinucleotide ic acid | Generator | | TPN | hmdb | | Triopyridine nucleotide | ChEBI | | Triphosphopyridine nucleotide | hmdb | | β-nicotinamide adenine dinucleotide ate | Generator | | β-nicotinamide adenine dinucleotide ic acid | Generator |
|
|---|
| Chemical Formula | C21H29N7O17P3 |
|---|
| IUPAC name | 1-[(2R,3R,4S,5R)-5-[({[({[(2R,3R,4R,5R)-5-(6-amino-9H-purin-9-yl)-3-hydroxy-4-(phosphonooxy)oxolan-2-yl]methoxy}(hydroxy)phosphoryl)oxy](hydroxy)phosphoryl}oxy)methyl]-3,4-dihydroxyoxolan-2-yl]-3-carbamoyl-1lambda5-pyridin-1-ylium |
|---|
| InChI Identifier | InChI=1S/C21H28N7O17P3/c22-17-12-19(25-7-24-17)28(8-26-12)21-16(44-46(33,34)35)14(30)11(43-21)6-41-48(38,39)45-47(36,37)40-5-10-13(29)15(31)20(42-10)27-3-1-2-9(4-27)18(23)32/h1-4,7-8,10-11,13-16,20-21,29-31H,5-6H2,(H7-,22,23,24,25,32,33,34,35,36,37,38,39)/p+1/t10-,11-,13-,14-,15-,16-,20-,21-/m1/s1 |
|---|
| InChI Key | XJLXINKUBYWONI-NNYOXOHSSA-O |
|---|
| Isomeric SMILES | NC(=O)C1=C[N+](=CC=C1)[C@@H]1O[C@H](CO[P@](O)(=O)O[P@](O)(=O)OC[C@H]2O[C@H]([C@H](OP(O)(O)=O)[C@@H]2O)N2C=NC3=C(N)N=CN=C23)[C@@H](O)[C@H]1O |
|---|
| Average Molecular Weight | 744.4129 |
|---|
| Monoisotopic Molecular Weight | 744.083277073 |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as l-alpha-amino acids. These are alpha amino acids which have the L-configuration of the alpha-carbon atom. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
| Direct Parent | L-alpha-amino acids |
|---|
| Alternative Parents | |
|---|
| Substituents | - L-alpha-amino acid
- Fatty acid
- Hydroxamic acid
- Amino acid
- Monocarboxylic acid or derivatives
- Carboxylic acid
- Amine
- Hydrocarbon derivative
- Organic oxygen compound
- Organic nitrogen compound
- Primary amine
- Organooxygen compound
- Organonitrogen compound
- Primary aliphatic amine
- Carbonyl group
- Organic oxide
- Organopnictogen compound
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Physico-Chemical Properties - Experimental |
|---|
| Property | Value | Reference |
|---|
| Experimental logP | Not Available | |
|---|
| Experimental Water Solubility | Not Available | |
|---|
| Melting Point | Not Available | |
|---|
|
| Foods of Origin |
|---|
| Food | Content Range | Average | Reference |
|---|
| Food | | | Reference |
|---|
|
| Production Data |
|---|
| Production Method | commercial |
|---|
| Production Method Reference | Not Available |
|---|
| Production Method Reference File | Not Available |
|---|
| Quantity Available | Production upon request, up to 1 g |
|---|
| Delivery Time | Not Available |
|---|
| Storage Form | solid |
|---|
| Storage Conditions | -80°C |
|---|
| Stability | Not Available |
|---|
| Purity | Not Available |
|---|
| Spectra |
|---|
| Spectral Data Upon Request | Not Available |
|---|
| Provider Information |
|---|
|
| Commercial Vendors |
|---|
| AKSci | M045 |
|---|
| Cayman Chemical | 21045 |
|---|
| MetaSci | HMDB0000217 |
|---|