| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation date | 2015-10-09 22:29:01 UTC |
|---|
| Update date | 2017-01-19 02:36:15 UTC |
|---|
| FoodComEx ID | PC000168 |
|---|
| FoodDB Record | FDB022253 |
|---|
| Chemical Information |
|---|
| Name | beta-N-Acetylglucosamine |
|---|
| Description | All animals and plants dynamically attach and remove beta-N-acetylglucosamine at serine and threonine residues on myriad nuclear and cytoplasmic proteins. b-N-Acetylglucosamine cycling, which is tightly regulated by the concerted actions of two highly conserved enzymes, serves as a nutrient and stress sensor. On some proteins, b-N-Acetylglucosamine competes directly with phosphate for serine/threonine residues. b-N-Acetylglucosamine's subcellular localization in hepatocytes established that it is highly concentrated at the nuclear envelope, particularly at the nuclear pore complex, but is also abundant and widespread within chromatin. However, several cytosolic and cytoskeletal proteins are also found to be glycosylated with b-N-Acetylglucosamine. b-N-Acetylglucosamination is in many ways distinct from 'classical' protein glycosylation. First, it is found mostly within the cytoplasm or nucleoplasm. Second, unlike the extraordinarily complex array of glycans found on extracellular glycoproteins, b-N-Acetylglucosamine is not elongated or further modified. Third, b-N-Acetylglucosamine cycles by means of mechanisms and on a timescale similar to those of phosphorylation and quite different from the cycling of complex extracellular glycans. b-N-Acetylglucosamination is one of the most common post-translational modifications. In terms of high-energy compounds, the intracellular concentration of the direct donor for b-N-Acetylglucosamination, UDP-GlcNAc, is second only to that of ATP, with 2-5% of all glucose being used to generate this sugar nucleotide. b-N-Acetylglucosaminated proteins can be found in almost every intracellular compartment, and there are proteins in almost every functional class that are subject to b-N-Acetylglucosamination. (PMID: 17460662) [HMDB] |
|---|
| CAS Number | 14131-68-1 |
|---|
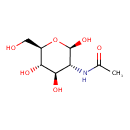
| Structure | |
|---|
| Synonyms | | Synonym | Source |
|---|
| 2-Acetamido-2-deoxy-b-D-glucose | hmdb | | 2-Acetamido-2-deoxy-beta-D-glucose | hmdb | | 2-Acetamido-2-deoxy-beta-delta-glucose | hmdb | | b-N-Acetyl-D-glucosamine | hmdb | | b-N-Acetylglucosamine | hmdb | | beta-N-Acetyl-D-glucosamine | hmdb | | beta-N-Acetyl-delta-glucosamine | hmdb | | beta-N-Acetylglucosamine | hmdb | | BetaGlcNAc | ChEBI | | GlcNAc-b | Generator | | GlcNAc-beta | ChEBI | | GlcNAc-β | Generator | | N-Acetyl-b-D-glucosamine | hmdb | | N-Acetyl-beta-D-glucosamine | hmdb | | N-Acetyl-β-D-glucosamine | Generator |
|
|---|
| Chemical Formula | C8H15NO6 |
|---|
| IUPAC name | N-[(2R,3R,4R,5S,6R)-2,4,5-trihydroxy-6-(hydroxymethyl)oxan-3-yl]acetamide |
|---|
| InChI Identifier | InChI=1S/C8H15NO6/c1-3(11)9-5-7(13)6(12)4(2-10)15-8(5)14/h4-8,10,12-14H,2H2,1H3,(H,9,11)/t4-,5-,6-,7-,8-/m1/s1 |
|---|
| InChI Key | OVRNDRQMDRJTHS-FMDGEEDCSA-N |
|---|
| Isomeric SMILES | CC(=O)N[C@H]1[C@H](O)O[C@H](CO)[C@@H](O)[C@@H]1O |
|---|
| Average Molecular Weight | 221.2078 |
|---|
| Monoisotopic Molecular Weight | 221.089937217 |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as acylaminosugars. These are organic compounds containing a sugar linked to a chain through N-acyl group. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic oxygen compounds |
|---|
| Class | Organooxygen compounds |
|---|
| Sub Class | Carbohydrates and carbohydrate conjugates |
|---|
| Direct Parent | Acylaminosugars |
|---|
| Alternative Parents | |
|---|
| Substituents | - Acylaminosugar
- N-acyl-alpha-hexosamine
- Hexose monosaccharide
- Monosaccharide
- Oxane
- Acetamide
- Carboxamide group
- Hemiacetal
- Secondary carboxylic acid amide
- Secondary alcohol
- Carboxylic acid derivative
- Oxacycle
- Organoheterocyclic compound
- Polyol
- Alcohol
- Hydrocarbon derivative
- Organic oxide
- Organic nitrogen compound
- Organopnictogen compound
- Organonitrogen compound
- Primary alcohol
- Carbonyl group
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Physico-Chemical Properties - Experimental |
|---|
| Property | Value | Reference |
|---|
| Experimental logP | Not Available | |
|---|
| Experimental Water Solubility | Not Available | |
|---|
| Melting Point | Not Available | |
|---|
|
| Foods of Origin |
|---|
| Food | Content Range | Average | Reference |
|---|
| Food | | | Reference |
|---|
|
| Production Data |
|---|
| Production Method | commercial |
|---|
| Production Method Reference | Not Available |
|---|
| Production Method Reference File | Not Available |
|---|
| Quantity Available | Production upon request, up to 1 g |
|---|
| Delivery Time | Not Available |
|---|
| Storage Form | solid |
|---|
| Storage Conditions | -80°C |
|---|
| Stability | Not Available |
|---|
| Purity | Not Available |
|---|
| Spectra |
|---|
| Spectral Data Upon Request | Not Available |
|---|
| Provider Information |
|---|
|
| Commercial Vendors |
|---|
| Not Available |