| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation date | 2015-10-09 22:29:00 UTC |
|---|
| Update date | 2017-01-19 02:36:15 UTC |
|---|
| FoodComEx ID | PC000167 |
|---|
| FoodDB Record | FDB000753 |
|---|
| Chemical Information |
|---|
| Name | Ethanol |
|---|
| Description | Ethanol, also known as ethyl alcohol or alcohol, belongs to the class of organic compounds known as primary alcohols. Primary alcohols are compounds having a primary alcohol functional group, with the general structure RCOH (R=alkyl, aryl). Ethanol is a clear, colorless liquid rapidly absorbed from the gastrointestinal tract and distributed throughout the body. It has bactericidal activity and is used often as a topical disinfectant. It is widely used as a solvent and preservative in pharmaceutical preparations as well as serving as the primary ingredient in alcoholic beverages. Indeed, ethanol has widespread use as a solvent of substances intended for human contact or consumption, including scents, flavorings, colorings, and medicines. Ethanol has a depressive effect on the central nervous system and because of its psychoactive effects, it is considered a drug. Ethanol has a complex mode of action and affects multiple systems in the brain, most notably it acts as an agonist to the GABA receptors. Death from ethanol consumption is possible when blood alcohol level reaches 0.4%. A blood level of 0.5% or more is commonly fatal. Levels of even less than 0.1% can cause intoxication, with unconsciousness often occurring at 0.3-0.4 %. Ethanol is metabolized by the body as an energy-providing carbohydrate nutrient, as it metabolizes into acetyl CoA, an intermediate common with glucose metabolism, that can be used for energy in the citric acid cycle or for biosynthesis. Ethanol within the human body is converted into acetaldehyde by alcohol dehydrogenase and then into acetic acid by acetaldehyde dehydrogenase. The product of the first step of this breakdown, acetaldehyde, is more toxic than ethanol. Acetaldehyde is linked to most of the adverse clinical effects of alcohol. Ethanol has been shown to increase the risk of developing cirrhosis of the liver, multiple forms of cancer, and alcoholism. Industrially, ethanol is produced both as a petrochemical, through the hydration of ethylene, and biologically, by fermenting sugars with yeast. Small amounts of ethanol are endogenously produced by gut microflora through anaerobic fermentation. However, most ethanol detected in biofluids and tissues likely comes from consumption of alcoholic beverages. Absolute ethanol or anhydrous alcohol generally refers to purified ethanol, containing no more than one percent water. Absolute alcohol is not intended for human consumption. It often contains trace amounts of toxic benzene (used to remove water by azeotropic distillation). Consumption of this form of ethanol can be fatal over a short time period. Generally absolute or pure ethanol is used as a solvent for lab and industrial settings where water will disrupt a desired reaction. Pure ethanol is classed as 200 proof in the USA and Canada, equivalent to 175 degrees proof in the UK system. Ethanol is a general biomarker for the consumption of alcohol. Ethanol is also a metabolite of Hansenula and Saccharomyces (PMID: 14613880). |
|---|
| CAS Number | 64-17-5 |
|---|
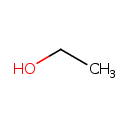
| Structure | |
|---|
| Synonyms | | Synonym | Source |
|---|
| [CH2Me(OH)] | ChEBI | | [OEtH] | ChEBI | | 1-hydroxyethane | manual | | Absolute alcohol | manual | | Absolute ethanol | HMDB | | Absolute ethyl alcohol | HMDB | | Aethanol | ChEBI | | Aethylalkohol | ChEBI | | Alcare hand degermer | HMDB | | Alcohol etilico | ChEBI | | Alcohols | HMDB | | Alcool ethylique | ChEBI | | Alcool etilico | HMDB | | Algrain | HMDB | | Alkohol | ChEBI | | Alkoholu etylowego | HMDB | | Anhydrol | HMDB | | Anhydrous alcohol | HMDB | | C2H5OH | ChEBI | | Cologne spirit | HMDB | | Cologne spirits | HMDB | | Dehydrated alcohol | HMDB | | Dehydrated ethanol | ChEBI | | Denatured ethanol | HMDB | | Desinfektol el | HMDB | | Diluted alcohol | HMDB | | Distilled spirits | HMDB | | Drinking alcohol | manual | | Etanol | ChEBI | | Ethanol 200 proof | HMDB | | Ethanol solution | HMDB | | Ethicap | HMDB | | Ethyl alc | HMDB | | Ethyl alcohol | db_source | | Ethyl alcohol anhydrous | HMDB | | Ethyl alcohol in alcoholic beverages | HMDB | | Ethyl alcohol usp | HMDB | | Ethyl hydrate | manual | | Ethyl hydroxide | manual | | Ethylic alcohol | manual | | Ethylol | manual | | EtOH | ChEBI | | FEMA 2419 | db_source | | Fermentation alcohol | HMDB | | Grain alcohol | manual | | Hinetoless | HMDB | | Hydroxyethane | db_source | | Infinity pure | HMDB | | Jaysol | HMDB | | Jaysol S | HMDB | | Lux | HMDB | | Methylcarbinol | manual | | Molasses alcohol | HMDB | | Potato alcohol | HMDB | | Punctilious ethyl alcohol | HMDB | | Pure alcohol | manual | | Pyro | HMDB | | Silent spirit | HMDB | | Spirit | HMDB | | Spirits OF wine | HMDB | | Spiritus vini | ChEBI | | Spirt | HMDB | | Synasol | HMDB | | Tecsol | HMDB | | Tecsol C | HMDB | | Thanol | HMDB | | Undenatured ethanol | HMDB |
|
|---|
| Chemical Formula | C2H6O |
|---|
| IUPAC name | ethanol |
|---|
| InChI Identifier | InChI=1S/C2H6O/c1-2-3/h3H,2H2,1H3 |
|---|
| InChI Key | LFQSCWFLJHTTHZ-UHFFFAOYSA-N |
|---|
| Isomeric SMILES | CCO |
|---|
| Average Molecular Weight | 46.0684 |
|---|
| Monoisotopic Molecular Weight | 46.041864814 |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as primary alcohols. Primary alcohols are compounds comprising the primary alcohol functional group, with the general structure RCOH (R=alkyl, aryl). |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic oxygen compounds |
|---|
| Class | Organooxygen compounds |
|---|
| Sub Class | Alcohols and polyols |
|---|
| Direct Parent | Primary alcohols |
|---|
| Alternative Parents | |
|---|
| Substituents | - Hydrocarbon derivative
- Primary alcohol
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Physico-Chemical Properties - Experimental |
|---|
| Property | Value | Reference |
|---|
| Experimental logP | -0.31 | HANSCH,C ET AL. (1995) |
|---|
| Experimental Water Solubility | 1000 mg/mL at 25 oC | RIDDICK,JA et al. (1986) |
|---|
| Melting Point | Fp -117.3° (-112.3°) | DFC |
|---|
|
| Foods of Origin |
|---|
| Food | Content Range | Average | Reference |
|---|
| Food | | | Reference |
|---|
|
| Production Data |
|---|
| Production Method | commercial |
|---|
| Production Method Reference | Not Available |
|---|
| Production Method Reference File | Not Available |
|---|
| Quantity Available | Production upon request, up to 100 mg |
|---|
| Delivery Time | Not Available |
|---|
| Storage Form | liquid |
|---|
| Storage Conditions | -80°C |
|---|
| Stability | Not Available |
|---|
| Purity | Not Available |
|---|
| Spectra |
|---|
| Spectral Data Upon Request | Not Available |
|---|
| Provider Information |
|---|
|
| Commercial Vendors |
|---|
| Toronto Research Chemicals | KIT1410 |
|---|