| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation date | 2015-10-09 22:28:58 UTC |
|---|
| Update date | 2017-01-19 02:36:15 UTC |
|---|
| FoodComEx ID | PC000163 |
|---|
| FoodDB Record | FDB003383 |
|---|
| Chemical Information |
|---|
| Name | 2-Butanone |
|---|
| Description | 2-Butanone or Butanone, also known as methylethylketone or MEK, belongs to the class of organic compounds known as ketones. It is an organic compound with the formula CH3C(O)CH2CH3. Ketones are organic compounds in which a carbonyl group is bonded to two carbon atoms R2C=O (neither R may be a hydrogen atom). Ketones that have one or more alpha-hydrogen atoms undergo keto-enol tautomerization, the tautomer being an enol. Thus, butanone is considered to be an oxygenated hydrocarbon lipid molecule. Butanone is a very hydrophobic molecule, practically insoluble in water, and relatively neutral. It has been isolated from hop oil (Humulus lupulus), white clover (Trifolium repens), tea, tomatoes, various fruits and other vegetable sources. It is used in the refining and extraction of fats and oils. Butanone is an irritant, but serious health effects in animals have been seen only at very high levels. When inhaled, these effects included birth defects. Butanone occurs as a natural product. It is produced by some trees and found in some fruits and vegetables in small amounts. It is also released to the air from car and truck exhausts. The known health effects to people from exposure to butanone are irritation of the nose, throat, skin, and eyes. This colorless liquid has a sharp, sweet odor, reminiscent of butterscotch and acetone. It is produced industrially at a large scale, and also occurs in trace amounts in nature. |
|---|
| CAS Number | 78-93-3 |
|---|
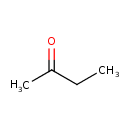
| Structure | |
|---|
| Synonyms | | Synonym | Source |
|---|
| 2-butanal | biospider | | 2-Butanon | biospider | | 2-butanone (MEK | biospider | | 2-butanone (MEK) | biospider | | 2-Oxobutane | db_source | | 3-Butanone | biospider | | Acetone, methyl- | biospider | | Aethylmethylketon | biospider | | butan-2-one | biospider | | Butanone | biospider | | Butanone 2 | biospider | | C2H5COCH3 | biospider | | Ethyl methyl cetone | ChEBI | | Ethyl methyl cetone | biospider | | Ethyl methyl ketone | db_source | | Ethyl(methyl) ketone | ChEBI | | Ethylmethyl ketone | ChEBI | | Ethylmethylcetone | biospider | | Ethylmethylketon | biospider | | Ethylmethylketone | biospider | | FEMA 2170 | db_source | | ghl.PD_Mitscher_leg0.417 | biospider | | HSDB 99 | biospider | | Ketone, ethyl methyl | biospider | | Ketone, methyl ethyl | biospider | | Meetco | biospider | | MEK | db_source | | Meketone | biospider | | Methyl acetone | biospider | | Methyl ethyl cetone | biospider | | Methyl ethyl ketone | db_source | | Methyl ethyl ketone) | biospider | | Methyl(ethyl) ketone | ChEBI | | Methylacetone | biospider | | Methylethyl ketone | biospider | | Methylethylketon | biospider | | Methylethylketone | biospider | | Metiletilcetona | biospider | | Metiletilchetone | biospider | | Metyl ethyl ketone | biospider | | Metyloetyloketon | biospider | | Oxobutane | biospider |
|
|---|
| Chemical Formula | C4H8O |
|---|
| IUPAC name | butan-2-one |
|---|
| InChI Identifier | InChI=1S/C4H8O/c1-3-4(2)5/h3H2,1-2H3 |
|---|
| InChI Key | ZWEHNKRNPOVVGH-UHFFFAOYSA-N |
|---|
| Isomeric SMILES | CCC(C)=O |
|---|
| Average Molecular Weight | 72.1057 |
|---|
| Monoisotopic Molecular Weight | 72.057514878 |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as ketones. These are organic compounds in which a carbonyl group is bonded to two carbon atoms R2C=O (neither R may be a hydrogen atom). Ketones that have one or more alpha-hydrogen atoms undergo keto-enol tautomerization, the tautomer being an enol. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic oxygen compounds |
|---|
| Class | Organooxygen compounds |
|---|
| Sub Class | Carbonyl compounds |
|---|
| Direct Parent | Ketones |
|---|
| Alternative Parents | |
|---|
| Substituents | - Ketone
- Organic oxide
- Hydrocarbon derivative
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Physico-Chemical Properties - Experimental |
|---|
| Property | Value | Reference |
|---|
| Experimental logP | 0.29 | HANSCH,C ET AL. (1995) |
|---|
| Experimental Water Solubility | 223 mg/mL at 25 oC | TAFT,RW et al. (1985) |
|---|
| Melting Point | Fp -85.9° | DFC |
|---|
|
| Foods of Origin |
|---|
| Food | Content Range | Average | Reference |
|---|
| Food | | | Reference |
|---|
|
| Production Data |
|---|
| Production Method | commercial |
|---|
| Production Method Reference | Not Available |
|---|
| Production Method Reference File | Not Available |
|---|
| Quantity Available | Production upon request, up to 300 mg |
|---|
| Delivery Time | Not Available |
|---|
| Storage Form | liquid |
|---|
| Storage Conditions | -80°C |
|---|
| Stability | Not Available |
|---|
| Purity | Not Available |
|---|
| Spectra |
|---|
| Spectral Data Upon Request | Not Available |
|---|
| Provider Information |
|---|
|
| Commercial Vendors |
|---|
| Toronto Research Chemicals | B689480 |
|---|