| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation date | 2015-10-09 22:28:57 UTC |
|---|
| Update date | 2017-01-19 02:36:15 UTC |
|---|
| FoodComEx ID | PC000162 |
|---|
| FoodDB Record | FDB012589 |
|---|
| Chemical Information |
|---|
| Name | Dimethylamine |
|---|
| Description | Dimethylamine, also known as DMA or (CH3)2NH, belongs to the class of organic compounds known as dialkylamines. These are organic compounds containing a dialkylamine group, characterized by two alkyl groups bonded to the amino nitrogen. Dimethylamine is a very strong basic compound (based on its pKa). Dimethylamine (DMA) is also classified as a secondary amine. It is a colorless, liquefied and flammable gas with an ammonia and fish-like odor. Dimethylamine is abundantly present in human urine. The main sources of urinary DMA have been reported to include trimethylamine N-oxide, a common food component found in cold-water fish, and asymmetric dimethylarginine (ADMA), an endogenous inhibitor of nitric oxide (NO) synthesis. ADMA is excreted in the urine in part unmetabolized and in part after hydrolysis to DMA by dimethylarginine dimethylaminohydrolase (DDAH). Statistically significant increases in urinary DMA have been found in individuals after the consumption of fish and seafoods. The highest values were obtained for individuals that consumed coley, squid, whitting, cod, haddock, sardine, skate and swordfish (PMID: 18282650). DMA has also been identified as a uremic toxin according to the European Uremic Toxin Working Group (PMID: 22626821). Industrially, DMA is used as dehairing agent in tanning, in dyes, in rubber polymerization accelerators, in soaps and cleaning compounds and as an agricultural fungicide. In the body, DMA also undergoes nitrosation under weak acid conditions to give dimethlynitrosamine. Recent studies have shown that DMA is a metabolite produced by several species of bacteria including Arthrobacter and Micrococcus (PMID: 11422368). Dimethylamine is also found in cannabis smoke and is volatilized during the combustion of cannabis (https://doi.org/10.1007/978-1-59259-947-9_2). |
|---|
| CAS Number | 124-40-3 |
|---|
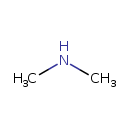
| Structure | |
|---|
| Synonyms | | Synonym | Source |
|---|
| (CH3)2NH | biospider | | Dimethylamine (anhydrous) | biospider | | Dimethylamine anhydrous | biospider | | Dimethylamine anhydrous (dot) | biospider | | Dimethylamine aqueous solution | HMDB | | Dimethylamine hydrobromide | biospider | | Dimethylamine solution | HMDB | | Dimethylamine, anhydrous [UN1032] [Flammable gas] | biospider | | Dimethylammonium bromide | biospider | | HNMe2 | biospider | | Me2NH | biospider | | Methanamine, n-methyl- | biospider | | Methanamine, n-methyl-, hydrobromide | biospider | | N-methyl-methanamine | biospider | | N-methylmethanamine | biospider | | N-Methylmethanamine (acd/name 4.0) | HMDB | | N-Methylmethanamine, 9CI | db_source | | N,n-dimethylamine | biospider | | nchem.125-comp12 | biospider |
|
|---|
| Chemical Formula | C2H7N |
|---|
| IUPAC name | dimethylamine |
|---|
| InChI Identifier | InChI=1S/C2H7N/c1-3-2/h3H,1-2H3 |
|---|
| InChI Key | ROSDSFDQCJNGOL-UHFFFAOYSA-N |
|---|
| Isomeric SMILES | CNC |
|---|
| Average Molecular Weight | 45.0837 |
|---|
| Monoisotopic Molecular Weight | 45.057849229 |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as dialkylamines. These are organic compounds containing a dialkylamine group, characterized by two alkyl groups bonded to the amino nitrogen. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic nitrogen compounds |
|---|
| Class | Organonitrogen compounds |
|---|
| Sub Class | Amines |
|---|
| Direct Parent | Dialkylamines |
|---|
| Alternative Parents | |
|---|
| Substituents | - Secondary aliphatic amine
- Organopnictogen compound
- Hydrocarbon derivative
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Physico-Chemical Properties - Experimental |
|---|
| Property | Value | Reference |
|---|
| Experimental logP | -0.38 | HANSCH,C ET AL. (1995) |
|---|
| Experimental Water Solubility | 1630 mg/mL at 40 oC | SCHWEIZER,AE et al. (1978) |
|---|
| Melting Point | Mp -96° | DFC |
|---|
|
| Foods of Origin |
|---|
| Food | Content Range | Average | Reference |
|---|
| Food | | | Reference |
|---|
|
| Production Data |
|---|
| Production Method | commercial |
|---|
| Production Method Reference | Not Available |
|---|
| Production Method Reference File | Not Available |
|---|
| Quantity Available | Production upon request, up to 2 g |
|---|
| Delivery Time | Not Available |
|---|
| Storage Form | liquid |
|---|
| Storage Conditions | -80°C |
|---|
| Stability | Not Available |
|---|
| Purity | Not Available |
|---|
| Spectra |
|---|
| Spectral Data Upon Request | Not Available |
|---|
| Provider Information |
|---|
|
| Commercial Vendors |
|---|
| Toronto Research Chemicals | D461480 |
|---|