| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation date | 2015-10-09 22:28:53 UTC |
|---|
| Update date | 2017-01-19 02:36:14 UTC |
|---|
| FoodComEx ID | PC000152 |
|---|
| FoodDB Record | FDB021992 |
|---|
| Chemical Information |
|---|
| Name | 17-Hydroxyprogesterone |
|---|
| Description | It serves as an intermediate in the biosynthesis of hydrocortisone and gonadal steroid hormones. It is derived from progesterone via 17-hydroxylase, a P450c17 enzyme, or from 17-hydroxypregnenolone via 3β-hydroxysteroid dehydrogenase/Δ5-4 isomerase. 17-Hydroxyprogesterone is a natural progestin and in pregnancy increases in the third trimester primarily due to fetal adrenal production.
This hormone is primarily produced in the adrenal glands and to some degree in the gonads, specifically the corpus luteum of the ovary. Normal levels are 3-90 ng/dl in children, and in women, 15-70 ng/dl prior to ovulation, and 35-290 ng/dl during the luteal phase.
Measurements of levels of 17-hydroxyprogesterone are useful in the evaluation of patients with suspected congenital adrenal hyperplasia as the typical enzymes that are defective, namely 21-hydroxylase and 11β-hydroxylase, lead to a build-up of 17OHP. In contrast, the rare patient with 17α-hydroxylase deficiency will have very low or undetectable levels of 17OHP. 17OHP levels can also be used to measure contribution of progestational activity of the corpus luteum during pregnancy as progesterone but not 17OHP is also contributed by the placenta. [HMDB] |
|---|
| CAS Number | 68-96-2 |
|---|
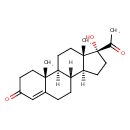
| Structure | |
|---|
| Synonyms | | Synonym | Source |
|---|
| 17-alpha-hydroxyprogesterone | hmdb | | 17-Hydroxypregn-4-en-3,20-dione | ChEBI | | 17-Hydroxypregn-4-ene-3,20-dione | hmdb | | 17-Hydroxyprogesterone | hmdb | | 17-OH Progesterone | hmdb | | 17-OHP | hmdb | | 17a-Hydroxy-4-pregnene-3,20-dione | Generator | | 17a-Hydroxy-progesterone | Generator | | 17a-Hydroxypregn-4-ene-3,20-dione | hmdb | | 17a-Hydroxyprogesterone | hmdb | | 17alpha-Hydroxy-4-pregnene-3,20-dione | ChEBI | | 17alpha-Hydroxy-progesterone | ChEBI | | 17alpha-hydroxyprogesterone | hmdb | | 17α-hydroxy-4-pregnene-3,20-dione | Generator | | 17α-hydroxy-progesterone | Generator | | 17α-hydroxyprogesterone | Generator | | D4-Pregnen-17a-ol-3,20-dione | hmdb | | delta(4)-Pregnene-17a-ol-3,20-dione | Generator | | delta(4)-Pregnene-17alpha-ol-3,20-dione | ChEBI | | Gestageno | hmdb | | Gestageno Gador | hmdb | | Hidroxiprogesterona | ChEBI | | Hydroxyprogesterone | hmdb | | Hydroxyprogesteronum | ChEBI | | Pregn-4-en-17a-ol-3,20-dione | hmdb | | Pregn-4-ene-3,20-dione-17-ol | ChEBI | | Prodix | hmdb | | Prodox | hmdb | | δ(4)-pregnene-17a-ol-3,20-dione | Generator | | δ(4)-pregnene-17α-ol-3,20-dione | Generator |
|
|---|
| Chemical Formula | C21H30O3 |
|---|
| IUPAC name | (1S,2R,10R,11S,14R,15S)-14-acetyl-14-hydroxy-2,15-dimethyltetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadec-6-en-5-one |
|---|
| InChI Identifier | InChI=1S/C21H30O3/c1-13(22)21(24)11-8-18-16-5-4-14-12-15(23)6-9-19(14,2)17(16)7-10-20(18,21)3/h12,16-18,24H,4-11H2,1-3H3/t16-,17+,18+,19+,20+,21+/m1/s1 |
|---|
| InChI Key | DBPWSSGDRRHUNT-CEGNMAFCSA-N |
|---|
| Isomeric SMILES | [H][C@@]12CC[C@](O)(C(C)=O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CCC2=CC(=O)CC[C@]12C |
|---|
| Average Molecular Weight | 330.4611 |
|---|
| Monoisotopic Molecular Weight | 330.219494826 |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as gluco/mineralocorticoids, progestogins and derivatives. These are steroids with a structure based on a hydroxylated prostane moiety. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Steroids and steroid derivatives |
|---|
| Sub Class | Pregnane steroids |
|---|
| Direct Parent | Gluco/mineralocorticoids, progestogins and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Progestogin-skeleton
- 20-oxosteroid
- 3-oxo-delta-4-steroid
- 3-oxosteroid
- 17-hydroxysteroid
- Oxosteroid
- Hydroxysteroid
- Delta-4-steroid
- Cyclohexenone
- Alpha-hydroxy ketone
- Cyclic alcohol
- Tertiary alcohol
- Cyclic ketone
- Ketone
- Organic oxygen compound
- Carbonyl group
- Hydrocarbon derivative
- Alcohol
- Organooxygen compound
- Organic oxide
- Aliphatic homopolycyclic compound
|
|---|
| Molecular Framework | Aliphatic homopolycyclic compounds |
|---|
| External Descriptors | - 17alpha-hydroxy steroid (CHEBI:17252 )
- Progestagens (C01176 )
- C21 steroids (gluco/mineralocorticoids, progestogins) and derivatives (LMST02030161 )
|
|---|
| Physico-Chemical Properties - Experimental |
|---|
| Property | Value | Reference |
|---|
| Experimental logP | Not Available | |
|---|
| Experimental Water Solubility | Not Available | |
|---|
| Melting Point | Not Available | |
|---|
|
| Foods of Origin |
|---|
| Food | Content Range | Average | Reference |
|---|
| Food | | | Reference |
|---|
|
| Production Data |
|---|
| Production Method | commercial |
|---|
| Production Method Reference | Not Available |
|---|
| Production Method Reference File | Not Available |
|---|
| Quantity Available | Production upon request, up to 1 g |
|---|
| Delivery Time | Not Available |
|---|
| Storage Form | solid |
|---|
| Storage Conditions | -80°C |
|---|
| Stability | Not Available |
|---|
| Purity | Not Available |
|---|
| Spectra |
|---|
| Spectral Data Upon Request | Not Available |
|---|
| Provider Information |
|---|
|
| Commercial Vendors |
|---|
| AKSci | H581 |
|---|
| AKSci | V1408 |
|---|
| MetaSci | HMDB0000374 |
|---|
| Tokyo Chemical Industry | HMDB0000374 |
|---|
| Toronto Research Chemicals | H952330 |
|---|