| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation date | 2015-10-09 22:28:47 UTC |
|---|
| Update date | 2017-01-19 02:36:14 UTC |
|---|
| FoodComEx ID | PC000138 |
|---|
| FoodDB Record | FDB012757 |
|---|
| Chemical Information |
|---|
| Name | Thymine 2-desoxyriboside |
|---|
| Description | Isolated from seedlings of Phaseolus vulgaris (kidney bean)
Deoxythymidine is non-toxic and as part of one of the four nucleotides in DNA it is a naturally occurring compound that exists in all living organisms and DNA viruses. RNA has uridine (uracil joined to ribose) instead. Uracil is chemically very similar to thymine, the latter being 5-methyluracil. Since thymine nucleotides are precursors of DNA, not RNA, the prefix "deoxy" is often left out, i.e., deoxythymidine is often just called thymidine.; In its composition, deoxythymidine is a nucleoside composed of deoxyribose (a pentose sugar) joined to the pyrimidine base thymine.; Iododeoxyuridine is a radiosensitizer and increases the amount of DNA damage received from ionizing radiation.; Thymidine (more precisely called deoxythymidine; can also be labelled deoxyribosylthymine, and thymine deoxyriboside) is a chemical compound, more precisely a pyrimidine deoxynucleoside. Deoxythymidine is the DNA nucleoside T, which pairs with deoxyadenosine (A) in double-stranded DNA. In cell biology it is used to synchronize the cells in S phase.; Thymidine is non-toxic and is a naturally occurring compound that exists in all living organisms and DNA viruses. 25% of DNA is composed of thymidine. RNA does not have thymidine and has uridine instead. Thymidine is a chemical compound which is a pyrimidine nucleoside. Thymidine is the DNA base T, which pairs with adenosine in double stranded DNA. Thymine 2-desoxyriboside is found in pulses, yellow wax bean, and green bean. |
|---|
| CAS Number | 50-89-5 |
|---|
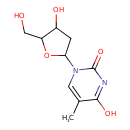
| Structure | |
|---|
| Synonyms | | Synonym | Source |
|---|
| (3H)Thymidine | biospider | | β-D-Ribofuranoside, thymine-1 2-deoxy- | biospider | | 1-(2-Deoxy-b-D-erythro-pentofuranosyl)-5-methyl-2,4(1H,3H)-pyrimidinedione | HMDB | | 1-(2-Deoxy-b-D-erythro-pentofuranosyl)-5-methyl-2,4(1H,3H)-pyrimidinedione, 9CI | db_source | | 1-(2-Deoxy-b-D-erythro-pentofuranosyl)-5-methylpyrimidine-2,4(1H,3H)-dione | Generator | | 1-(2-Deoxy-b-D-ribofuranosyl)-5-methyluracil | db_source | | 1-(2-Deoxy-beta-D-erythro-pentofuranosyl)-5-methylpyrimidine-2,4(1H,3H)-dione | ChEBI | | 1-(2-Deoxy-beta-D-ribofuranosyl)-5-methyluracil | biospider | | 1-(2-Deoxy-beta-D-ribofuranosyl)thymine | biospider | | 1-(2-Deoxy-beta-delta-erythro-pentofuranosyl)-5-methyl-2,4(1H,3H)-pyrimidinedione | HMDB | | 1-(2-Deoxy-beta-ribofuranosyl)-5-methyluracil | biospider | | 1-(2-Deoxy-β-D-erythro-pentofuranosyl)-5-methylpyrimidine-2,4(1H,3H)-dione | Generator | | 1-[4-Hydroxy-5-(hydroxymethyl)oxolan-2-yl]-5-methyl-pyrimidine-2,4-dione | HMDB | | 2-deoxythymidine | biospider | | 2-Desoxy-thymidine | biospider | | 2'-deoxy-5-methyl-Uridine | biospider | | 2'-deoxy-5-methyluridine | biospider | | 2'-Deoxythymidine | biospider | | 2'-thymidine | biospider | | 5-Methyl-2'-deoxyuridine | biospider | | 5-Methyldeoxyuridine | biospider | | 5-Methyldeoxyurindine | biospider | | beta-D-Ribofuranoside, thymine-1 2-deoxy- | biospider | | Deoxyribothymidine | biospider | | Deoxythymidin | biospider | | Deoxythymidine | biospider | | Desoxy-thymidin | biospider | | DT | biospider | | DTHD | biospider | | Dthyd | biospider | | NSC 21548 | db_source | | THM | biospider | | Thymidin | biospider | | Thymidine | ChEBI | | Thymidine (8CI,9CI) | biospider | | Thymidine-(H-3) | biospider | | Thymidine, 2'-deoxy- | biospider | | Thymidine, labeled with tritium | biospider | | Thymine 2-desoxyriboside | db_source | | thymine 2'-deoxyriboside | biospider | | Thymine deoxyriboside | biospider | | thymine-1 2-deoxy-b-D-Ribofuranoside | biospider | | thymine-1 2-deoxy-beta-delta-Ribofuranoside | biospider | | Thymine-2-deoxyriboside | biospider | | Thymine-2-desoxyriboside | biospider | | Thyminedeoxyriboside | biospider | | Thymosine | db_source | | Tritiated thymidine | biospider | | Uridine, 2'-deoxy-5-methyl- | biospider |
|
|---|
| Chemical Formula | C10H14N2O5 |
|---|
| IUPAC name | 4-hydroxy-1-[4-hydroxy-5-(hydroxymethyl)oxolan-2-yl]-5-methyl-1,2-dihydropyrimidin-2-one |
|---|
| InChI Identifier | InChI=1S/C10H14N2O5/c1-5-3-12(10(16)11-9(5)15)8-2-6(14)7(4-13)17-8/h3,6-8,13-14H,2,4H2,1H3,(H,11,15,16) |
|---|
| InChI Key | IQFYYKKMVGJFEH-UHFFFAOYSA-N |
|---|
| Isomeric SMILES | CC1=CN(C2CC(O)C(CO)O2)C(=O)N=C1O |
|---|
| Average Molecular Weight | 242.2286 |
|---|
| Monoisotopic Molecular Weight | 242.090271568 |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as pyrimidine 2'-deoxyribonucleosides. Pyrimidine 2'-deoxyribonucleosides are compounds consisting of a pyrimidine linked to a ribose which lacks a hydroxyl group at position 2. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Nucleosides, nucleotides, and analogues |
|---|
| Class | Pyrimidine nucleosides |
|---|
| Sub Class | Pyrimidine 2'-deoxyribonucleosides |
|---|
| Direct Parent | Pyrimidine 2'-deoxyribonucleosides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Pyrimidine 2'-deoxyribonucleoside
- Pyrimidone
- Hydropyrimidine
- Pyrimidine
- Heteroaromatic compound
- Tetrahydrofuran
- Vinylogous amide
- Lactam
- Secondary alcohol
- Urea
- Oxacycle
- Azacycle
- Organoheterocyclic compound
- Hydrocarbon derivative
- Organic oxide
- Organopnictogen compound
- Primary alcohol
- Organooxygen compound
- Organonitrogen compound
- Organic oxygen compound
- Alcohol
- Organic nitrogen compound
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Physico-Chemical Properties - Experimental |
|---|
| Property | Value | Reference |
|---|
| Experimental logP | -0.93 | SANGSTER (1993) |
|---|
| Experimental Water Solubility | Not Available | |
|---|
| Melting Point | Mp 186-187° | DFC |
|---|
|
| Foods of Origin |
|---|
| Food | Content Range | Average | Reference |
|---|
| Food | | | Reference |
|---|
|
| Production Data |
|---|
| Production Method | commercial |
|---|
| Production Method Reference | Not Available |
|---|
| Production Method Reference File | Not Available |
|---|
| Quantity Available | Production upon request, up to 20 mg |
|---|
| Delivery Time | Not Available |
|---|
| Storage Form | solid |
|---|
| Storage Conditions | -80°C |
|---|
| Stability | Not Available |
|---|
| Purity | Not Available |
|---|
| Spectra |
|---|
| Spectral Data Upon Request | Not Available |
|---|
| Provider Information |
|---|
|
| Commercial Vendors |
|---|
| AKSci | C514 |
|---|
| AKSci | J10255 |
|---|
| AKSci | J93106 |
|---|
| Cayman Chemical | 20519 |
|---|
| Glentham | GE6597 |
|---|
| MetaSci | HMDB0000273 |
|---|
| Sigma-Aldrich | HMDB0000273 |
|---|
| Toronto Research Chemicals | T412000 |
|---|