| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation date | 2015-10-09 22:28:42 UTC |
|---|
| Update date | 2017-01-19 02:36:13 UTC |
|---|
| FoodComEx ID | PC000129 |
|---|
| FoodDB Record | FDB012397 |
|---|
| Chemical Information |
|---|
| Name | L-Isoleucine |
|---|
| Description | Dietary supplement, nutrient
Branched chain amino acids (BCAA) are essential amino acids whose carbon structure is marked by a branch point. These three amino acids are critical to human life and are particularly involved in stress, energy and muscle metabolism. BCAA supplementation as therapy, both oral and intravenous, in human health and disease holds great promise. "BCAA" denotes valine, isoleucine and leucine which are branched chain essential amino acids. Despite their structural similarities, the branched amino acids have different metabolic routes, with valine going solely to carbohydrates, leucine solely to fats and isoleucine to both. The different metabolism accounts for different requirements for these essential amino acids in humans: 12 mg/kg, 14 mg/kg and 16 mg/kg of valine, leucine and isoleucine respectively. Furthermore, these amino acids have different deficiency symptoms. Valine deficiency is marked by neurological defects in the brain, while isoleucine deficiency is marked by muscle tremors. BCAA are decreased in patients with liver disease, such as hepatitis, hepatic coma, cirrhosis, extrahepatic biliary atresia or portacaval shunt; They provide ingredients for the manufacturing of other essential biochemical components in the body, some of which are utilized for the production of energy, stimulants to the upper brain and helping you to be more alert.; aromatic amino acids (AAA)-tyrosine, tryptophan and phenylalanine, as well as methionine-are increased in these conditions. Valine, in particular, has been established as a useful supplemental therapy to the ailing liver. All the BCAA probably compete with AAA for absorption into the brain. Supplemental BCAA with vitamin B6 and zinc help normalize the BCAA:AAA ratio. The BCAA are not without side effects. Leucine alone, for example, exacerbates pellagra and can cause psychosis in pellagra patients by increasing excretion of niacin in the urine. Leucine may lower brain serotonin and dopamine. A dose of 3 g of isoleucine added to the niacin regime has cleared leucine-aggravated psychosis in schizophrenic patients. Isoleucine may have potential as an antipsychotic treatment. Leucine is more highly concentrated in foods than other amino acids. A cup of milk contains 800 mg of leucine and only 500 mg of isoleucine and valine. A cup of wheat germ has about 1.6 g of leucine and 1 g of isoleucine and valine. The ratio evens out in eggs and cheese. One egg and an ounce of most cheeses each contain about 400 mg of leucine and 400 mg of valine and isoleucine. The ratio of leucine to other BCAA is greatest in pork, where leucine is 7 to 8 g and the other BCAA together are only 3 to 4 g. (http://www.dcnutrition.com). L-Isoleucine is found in many foods, some of which are atlantic salmon, quince, baked potato, and pistachio. |
|---|
| CAS Number | 73-32-5 |
|---|
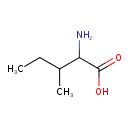
| Structure | |
|---|
| Synonyms | | Synonym | Source |
|---|
| (2S,3S)-2-amino-3-Methyl-pentanoate | HMDB | | (2S,3S)-2-amino-3-Methyl-pentanoic acid | HMDB | | (2S,3S)-2-amino-3-Methylpentanoate | Generator | | (2S,3S)-2-amino-3-Methylpentanoic acid | ChEBI | | (2S,3S)-a-amino-b-Methyl-N-valerate | HMDB | | (2S,3S)-a-amino-b-Methyl-N-valeric acid | HMDB | | (2S,3S)-a-amino-b-Methylvalerate | HMDB | | (2S,3S)-a-amino-b-Methylvaleric acid | HMDB | | (2S,3S)-Alph-amino-beta-methylvalerate | HMDB | | (2S,3S)-Alph-amino-beta-methylvaleric acid | HMDB | | (2S,3S)-alpha-amino-beta-Merthyl-N-valerate | HMDB | | (2S,3S)-alpha-amino-beta-Merthyl-N-valeric acid | HMDB | | (2S,3S)-alpha-amino-beta-Merthylvalerate | HMDB | | (2S,3S)-alpha-amino-beta-Merthylvaleric acid | HMDB | | (2S,3S)-alpha-amino-beta-Methyl-N-valerate | HMDB | | (2S,3S)-alpha-amino-beta-Methyl-N-valeric acid | HMDB | | (2S,3S)-alpha-amino-beta-Methylvalerate | HMDB | | (2S,3S)-alpha-amino-beta-Methylvaleric acid | HMDB | | (S,S)-Isoleucine | HMDB | | (S)-Isoleucine | HMDB | | [S-(R*,r*)]-2-amino-3-methylpentanoate | HMDB | | [S-(R*,r*)]-2-amino-3-methylpentanoic acid | HMDB | | α-amino-β-methylvaleric acid | biospider | | 2-amino-3-Methylpentanoate | HMDB | | 2-amino-3-Methylpentanoic acid | HMDB | | 2-amino-3-Methylvalerate | Generator | | 2-amino-3-Methylvaleric acid | ChEBI | | 2S,3S-Isoleucine | HMDB | | a-amino-b-Methylvalerate | Generator | | a-amino-b-Methylvaleric acid | Generator | | alpha-amino-beta-Methylvalerate | Generator | | alpha-amino-beta-Methylvaleric acid | ChEBI | | erythro-L-Isoleucine | HMDB | | Ile | ChEBI | | Iso-leucine | HMDB | | Isoleucine (van) | biospider | | Isoleucine; L-erythro-form | db_source | | L-(+)-Isoleucine | HMDB | | L-Ile | HMDB | | L-Isoleucine | db_source | | sec-C4H9CH(NH2)COOH | biospider | | α-amino-β-methylvalerate | Generator | | α-amino-β-methylvaleric acid | Generator |
|
|---|
| Chemical Formula | C6H13NO2 |
|---|
| IUPAC name | 2-amino-3-methylpentanoic acid |
|---|
| InChI Identifier | InChI=1S/C6H13NO2/c1-3-4(2)5(7)6(8)9/h4-5H,3,7H2,1-2H3,(H,8,9) |
|---|
| InChI Key | AGPKZVBTJJNPAG-UHFFFAOYSA-N |
|---|
| Isomeric SMILES | CCC(C)C(N)C(O)=O |
|---|
| Average Molecular Weight | 131.1729 |
|---|
| Monoisotopic Molecular Weight | 131.094628665 |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as isoleucine and derivatives. Isoleucine and derivatives are compounds containing isoleucine or a derivative thereof resulting from reaction of isoleucine at the amino group or the carboxy group, or from the replacement of any hydrogen of glycine by a heteroatom. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
| Direct Parent | Isoleucine and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Isoleucine or derivatives
- Alpha-amino acid
- Branched fatty acid
- Methyl-branched fatty acid
- Fatty acid
- Fatty acyl
- Amino acid
- Carboxylic acid
- Monocarboxylic acid or derivatives
- Organopnictogen compound
- Primary amine
- Organooxygen compound
- Organonitrogen compound
- Organic nitrogen compound
- Primary aliphatic amine
- Carbonyl group
- Organic oxygen compound
- Amine
- Organic oxide
- Hydrocarbon derivative
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Physico-Chemical Properties - Experimental |
|---|
| Property | Value | Reference |
|---|
| Experimental logP | -1.70 | HANSCH,C ET AL. (1995) |
|---|
| Experimental Water Solubility | 34.4 mg/mL at 25 oC | YALKOWSKY,SH & DANNENFELSER,RM (1992) |
|---|
| Melting Point | Mp 285-286° dec. | DFC |
|---|
|
| Foods of Origin |
|---|
| Food | Content Range | Average | Reference |
|---|
| Food | | | Reference |
|---|
|
| Production Data |
|---|
| Production Method | commercial |
|---|
| Production Method Reference | Not Available |
|---|
| Production Method Reference File | Not Available |
|---|
| Quantity Available | Production upon request, up to 8 g |
|---|
| Delivery Time | Not Available |
|---|
| Storage Form | solid |
|---|
| Storage Conditions | -80°C |
|---|
| Stability | Not Available |
|---|
| Purity | Not Available |
|---|
| Spectra |
|---|
| Spectral Data Upon Request | Not Available |
|---|
| Provider Information |
|---|
|
| Commercial Vendors |
|---|
| AKSci | G141 |
|---|
| AKSci | J93639 |
|---|
| Glentham | GM6591 |
|---|
| Glentham | GM8967 |
|---|
| MetaSci | HMDB0000172 |
|---|
| Sigma-Aldrich | HMDB0000172 |
|---|
| Toronto Research Chemicals | I820175 |
|---|