| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation date | 2015-10-09 22:28:41 UTC |
|---|
| Update date | 2017-01-19 02:36:13 UTC |
|---|
| FoodComEx ID | PC000128 |
|---|
| FoodDB Record | FDB003632 |
|---|
| Chemical Information |
|---|
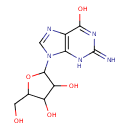
| Name | Guanosine |
|---|
| Description | Guanosine is a nucleoside comprising guanine attached to a ribose (ribofuranose) ring via a beta-N9-glycosidic bond. Guanosine can be phosphorylated to become GMP (guanosine monophosphate), cGMP (cyclic guanosine monophosphate), GDP (guanosine diphosphate) and GTP (guanosine triphosphate). ; The nucleoside guanosine exert important neuroprotective and neuromodulator roles in the central nervous system, which may be related to inhibition of the glutamatergic neurotransmission activity. Guanosine is the specific extracellular guanine-based purines effector and indicate that its conversion occurs not only in the central nervous system but also peripherally. (PMID: 16325434); Guanosine is a nucleoside comprising guanine attached to a ribose (ribofuranose) ring via a ?-N9-glycosidic bond. Guanosine is found in many foods, some of which are elderberry, malus (crab apple), acerola, and arrowhead. |
|---|
| CAS Number | 118-00-3 |
|---|
| Structure | |
|---|
| Synonyms | | Synonym | Source |
|---|
| 2-amino-1,9-dihydro-9-b-D-Ribofuranosyl-6H-purin-6-one | Generator | | 2-Amino-1,9-dihydro-9-beta-D-ribofuranosyl-6H-purin-6-one | biospider | | 2-amino-1,9-dihydro-9-beta-delta-Ribofuranosyl-6H-purin-6-one | HMDB | | 2-amino-1,9-dihydro-9-β-D-ribofuranosyl-6H-purin-6-one | Generator | | 2-amino-9-b-D-Ribofuranosyl-1,9-dihydro-6H-purin-6-one | Generator | | 2-amino-9-beta-D-ribofuranosyl-1,9-dihydro-6H-purin-6-one | biospider | | 2-amino-9-β-D-ribofuranosyl-1,9-dihydro-6H-purin-6-one | Generator | | 2-amino-Inosine | biospider | | 2(3H)-Imino-9-b-D-ribofuranosyl-9H-purin-6(1H)-one | Generator | | 2(3H)-Imino-9-beta-D-ribofuranosyl-9H-purin-6(1H)-one | biospider | | 2(3H)-Imino-9-β-D-ribofuranosyl-9H-purin-6(1H)-one | Generator | | 9-(beta-D-Ribofuranosyl)guanine | biospider | | 9-β-d-Arabinofuranosylguanine | biospider | | 9-b-D-ribofuranosyl-Guanine | biospider | | 9-beta-D-Ribofuranosyl-guanine | ChEBI | | 9-beta-D-Ribofuranosylguanine | biospider | | 9-beta-delta-ribofuranosyl-Guanine | biospider | | 9-β-D-ribofuranosyl-guanine | Generator | | Arabinosylguanine | biospider | | b-D-Ribofuranoside guanine-9 | biospider | | beta-delta-Ribofuranoside guanine-9 | biospider | | Guanine riboside | biospider | | Guanine-9-b-D-ribofuranoside | Generator | | Guanine-9-beta-D-ribofuranoside | biospider | | Guanine-9-β-D-ribofuranoside | Generator | | Guanosin | biospider | | Guo | ChEBI | | Nucleoside q | biospider | | Ribonucleoside | HMDB | | Vernine | biospider |
|
|---|
| Chemical Formula | C10H13N5O5 |
|---|
| IUPAC name | 2-(6-hydroxy-2-imino-3,9-dihydro-2H-purin-9-yl)-5-(hydroxymethyl)oxolane-3,4-diol |
|---|
| InChI Identifier | InChI=1S/C10H13N5O5/c11-10-13-7-4(8(19)14-10)12-2-15(7)9-6(18)5(17)3(1-16)20-9/h2-3,5-6,9,16-18H,1H2,(H3,11,13,14,19) |
|---|
| InChI Key | NYHBQMYGNKIUIF-UHFFFAOYSA-N |
|---|
| Isomeric SMILES | NC1=NC(=O)C2=C(N1)N(C=N2)C1OC(CO)C(O)C1O |
|---|
| Average Molecular Weight | 283.2407 |
|---|
| Monoisotopic Molecular Weight | 283.091668551 |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as purine nucleosides. Purine nucleosides are compounds comprising a purine base attached to a ribosyl or deoxyribosyl moiety. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Nucleosides, nucleotides, and analogues |
|---|
| Class | Purine nucleosides |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Purine nucleosides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Purine nucleoside
- Glycosyl compound
- N-glycosyl compound
- 6-oxopurine
- Hypoxanthine
- Pentose monosaccharide
- Purinone
- Imidazopyrimidine
- Purine
- Aminopyrimidine
- Pyrimidone
- Monosaccharide
- N-substituted imidazole
- Pyrimidine
- Azole
- Imidazole
- Heteroaromatic compound
- Vinylogous amide
- Tetrahydrofuran
- Secondary alcohol
- Organoheterocyclic compound
- Azacycle
- Oxacycle
- Organooxygen compound
- Organonitrogen compound
- Amine
- Alcohol
- Hydrocarbon derivative
- Organic oxide
- Organopnictogen compound
- Organic nitrogen compound
- Primary alcohol
- Primary amine
- Organic oxygen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Physico-Chemical Properties - Experimental |
|---|
| Property | Value | Reference |
|---|
| Experimental logP | -1.90 | SANGSTER (1993) |
|---|
| Experimental Water Solubility | 0.7 mg/mL at 18 oC | YALKOWSKY,SH & DANNENFELSER,RM (1992) |
|---|
| Melting Point | 239 oC | |
|---|
|
| Foods of Origin |
|---|
| Food | Content Range | Average | Reference |
|---|
| Food | | | Reference |
|---|
|
| Production Data |
|---|
| Production Method | commercial |
|---|
| Production Method Reference | Not Available |
|---|
| Production Method Reference File | Not Available |
|---|
| Quantity Available | Production upon request, up to 1 g |
|---|
| Delivery Time | Not Available |
|---|
| Storage Form | solid |
|---|
| Storage Conditions | -80°C |
|---|
| Stability | Not Available |
|---|
| Purity | Not Available |
|---|
| Spectra |
|---|
| Spectral Data Upon Request | Not Available |
|---|
| Provider Information |
|---|
|
| Commercial Vendors |
|---|
| AKSci | 2451AH |
|---|
| AKSci | I890 |
|---|
| Glentham | GE8690 |
|---|
| MetaSci | HMDB0000133 |
|---|
| Sigma-Aldrich | HMDB0000133 |
|---|
| Toronto Research Chemicals | G837900 |
|---|