| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation date | 2015-10-09 22:28:35 UTC |
|---|
| Update date | 2017-01-19 02:36:13 UTC |
|---|
| FoodComEx ID | PC000116 |
|---|
| FoodDB Record | FDB004222 |
|---|
| Chemical Information |
|---|
| Name | Guanine |
|---|
| Description | Guanine, also known as G or mearlmaid aa, belongs to the class of organic compounds known as purines and purine derivatives. These are aromatic heterocyclic compounds containing a purine moiety, which is formed a pyrimidine-ring ring fused to an imidazole ring. Guanine is a moderately basic compound (based on its pKa). Guanine exists in all living species, ranging from bacteria to humans. Within humans, guanine participates in a number of enzymatic reactions. In particular, guanine and phosphoribosyl pyrophosphate can be biosynthesized from guanosine monophosphate through its interaction with the enzyme adenine phosphoribosyltransferase. In addition, guanine and ribose 1-phosphate can be biosynthesized from guanosine; which is catalyzed by the enzyme purine nucleoside phosphorylase. In humans, guanine is involved in the metabolic disorder called the gout or kelley-seegmiller syndrome pathway. Outside of the human body, Guanine is found, on average, in the highest concentration within beers and milk (cow). Guanine has also been detected, but not quantified in, several different foods, such as kombus, black radish, chinese cabbages, dills, and devilfish. This could make guanine a potential biomarker for the consumption of these foods. Guanine, with regard to humans, has been found to be associated with several diseases such as lewy body disease, frontotemporal dementia, colorectal cancer, and alzheimer's disease; guanine has also been linked to the inborn metabolic disorder lesch-nyhan syndrome. A 2-aminopurine carrying a 6-oxo substituent. |
|---|
| CAS Number | 73-40-5 |
|---|
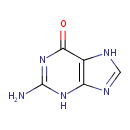
| Structure | |
|---|
| Synonyms | | Synonym | Source |
|---|
| 2-Amino-1,7-dihydro-6H-Purin-6-one | biospider | | 2-Amino-1,9-dihydro-6H-purin-6-one | biospider | | 2-Amino-1,9-dihydro-purin-6-one | biospider | | 2-Amino-3,7-dihydro-6H-purin-6-one | biospider | | 2-Amino-6-hydroxy-1H-purine | biospider | | 2-Amino-6-hydroxypurine | biospider | | 2-amino-6-Oxopurine | ChEBI | | 2-Amino-6-purinol | biospider | | 2-Amino-9H-purin-6-ol | biospider | | 2-Amino-Hypoxanthine | biospider | | 2-Aminohypoxanthine | biospider | | 2(1H)-pyrimidinone, 4-amino- | biospider | | 6-Hydroxy-2-aminopurine | biospider | | 6H-Purin-6-one, 2-amino-1,7-dihydro- (9CI) | biospider | | 6H-Purin-6-one, 2-amino-1,9-dihydro- | biospider | | 6H-purin-6-one, 2-amino-3,7-dihydro- | biospider | | C.I. natural white 1 | HMDB | | CI natural white 1 | HMDB | | Dew pearl | HMDB | | Gua | ChEBI | | Guanin | HMDB | | Guanine enol | HMDB | | GUN | HMDB | | Hypoxanthine, 2-amino- | biospider | | Mearlmaid | HMDB | | Mearlmaid aa | HMDB | | Natural pearl essence | HMDB | | Natural white 1 | HMDB | | Naturon | HMDB | | Pathocidin | HMDB | | Pearl essence | HMDB | | Stella polaris | HMDB |
|
|---|
| Chemical Formula | C5H5N5O |
|---|
| IUPAC name | 2-amino-6,7-dihydro-3H-purin-6-one |
|---|
| InChI Identifier | InChI=1S/C5H5N5O/c6-5-9-3-2(4(11)10-5)7-1-8-3/h1H,(H4,6,7,8,9,10,11) |
|---|
| InChI Key | UYTPUPDQBNUYGX-UHFFFAOYSA-N |
|---|
| Isomeric SMILES | NC1=NC(=O)C2=C(N1)N=CN2 |
|---|
| Average Molecular Weight | 151.1261 |
|---|
| Monoisotopic Molecular Weight | 151.049409807 |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as purines and purine derivatives. These are aromatic heterocyclic compounds containing a purine moiety, which is formed a pyrimidine-ring ring fused to an imidazole ring. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Imidazopyrimidines |
|---|
| Sub Class | Purines and purine derivatives |
|---|
| Direct Parent | Purines and purine derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Purine
- Hydroxypyrimidine
- Pyrimidine
- Heteroaromatic compound
- Imidazole
- Azole
- Azacycle
- Organic nitrogen compound
- Organic oxygen compound
- Organopnictogen compound
- Hydrocarbon derivative
- Organooxygen compound
- Organonitrogen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Physico-Chemical Properties - Experimental |
|---|
| Property | Value | Reference |
|---|
| Experimental logP | -0.91 | HANSCH,C ET AL. (1995) |
|---|
| Experimental Water Solubility | 2.08 mg/mL at 37 oC | YALKOWSKY,SH & DANNENFELSER,RM (1992) |
|---|
| Melting Point | Mp 300° | CCD |
|---|
|
| Foods of Origin |
|---|
| Food | Content Range | Average | Reference |
|---|
| Food | | | Reference |
|---|
|
| Production Data |
|---|
| Production Method | commercial |
|---|
| Production Method Reference | Not Available |
|---|
| Production Method Reference File | Not Available |
|---|
| Quantity Available | Production upon request, up to 3 g |
|---|
| Delivery Time | Not Available |
|---|
| Storage Form | solid |
|---|
| Storage Conditions | -80°C |
|---|
| Stability | Not Available |
|---|
| Purity | Not Available |
|---|
| Spectra |
|---|
| Spectral Data Upon Request | Not Available |
|---|
| Provider Information |
|---|
|
| Commercial Vendors |
|---|
| AKSci | A416 |
|---|
| AKSci | J20096 |
|---|
| AKSci | J92186 |
|---|
| AKSci | HMDB0000132 |
|---|
| Glentham | GE0617 |
|---|
| MetaSci | HMDB0000132 |
|---|
| Toronto Research Chemicals | G836000 |
|---|