| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation date | 2015-10-09 22:28:32 UTC |
|---|
| Update date | 2017-01-19 02:36:13 UTC |
|---|
| FoodComEx ID | PC000112 |
|---|
| FoodDB Record | FDB011999 |
|---|
| Chemical Information |
|---|
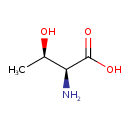
| Name | L-Threonine |
|---|
| Description | Threonine, abbreviated Thr or T, is an essential amino acid in humans. It has a side chain containing a hydroxyl group, making it a polar, uncharged amino acid Threonine is catabolized in mammals largely (70-80%) by threonine dehydrogenase (EC 1.1.1.103) that oxidizes threonine to 2-amino-3-oxobutyrate (which forms glycine and acetyl CoA), and much less by threonine dehydratase (EC 4.2.1.16) that catabolizes threonine into 2-oxobutyrate and ammonia. It is highly concentrated in meat products, cottage cheese and wheat germ. It is abundant in human plasma, particularly in newborns. The threonine content of most infant formulas currently on the market is approximately 20% higher than the threonine concentration in human milk. Premature infants fed these formulas have twice the plasma threonine concentrations of breast-fed infants. Increasing the threonine plasma concentrations leads to accumulation of threonine and glycine in the brain. Such accumulation affects the neurotransmitter balance which may have consequences for the brain development during early postnatal life. Thus, excessive threonine intake during infant feeding should be avoided (PMID 9853925). Threonine is an immunostimulant which promotes the growth of thymus gland and probably promotes cell immune defense function. This amino acid has been useful in the treatment of genetic spasticity disorders and multiple sclerosis at a dose of 1 g per day. Severe deficiency of threonine causes neurological dysfunction and lameness in experimental animals. |
|---|
| CAS Number | 72-19-5 |
|---|
| Structure | |
|---|
| Synonyms | | Synonym | Source |
|---|
| (2S,3R)-(-)-Threonine | biospider | | (2S,3R)-2-Amino-3-hydroxybutanoic acid | biospider | | (2S,3R)-2-Amino-3-hydroxybutyrate | biospider | | (2S,3R)-2-Amino-3-hydroxybutyric acid | biospider | | (2S)-Threonine | biospider | | (R-(R*,s*))-2-amino-3-hydroxybutanoate | HMDB | | (R-(R*,s*))-2-amino-3-hydroxybutanoic acid | HMDB | | (S)-Threonine | biospider | | [R-(R*,s*)]-2-amino-3-hydroxy-butanoate | HMDB | | [R-(R*,s*)]-2-amino-3-hydroxy-butanoic acid | HMDB | | [R-(R*,s*)]-2-amino-3-hydroxybutanoate | HMDB | | [R-(R*,s*)]-2-amino-3-hydroxybutanoic acid | HMDB | | 2-amino-3-Hydroxybutanoate | HMDB | | 2-amino-3-Hydroxybutanoic acid | HMDB | | 2-amino-3-Hydroxybutyrate | Generator | | 2-amino-3-Hydroxybutyric acid | ChEBI | | L-(-)-Threonine | biospider | | L-2-Amino-3-hydroxybutyrate | biospider | | L-2-Amino-3-hydroxybutyric acid | biospider | | L-a-amino-b-Hydroxybutyrate | Generator | | L-a-amino-b-Hydroxybutyric acid | Generator | | L-alpha-Amino-beta-hydroxybutyrate | biospider | | L-alpha-Amino-beta-hydroxybutyric acid | biospider | | L-Thr | biospider | | L-Threonin | ChEBI | | L-Threonine (9CI) | biospider | | L-α-amino-β-hydroxybutyrate | Generator | | L-α-amino-β-hydroxybutyric acid | Generator | | T | ChEBI | | Thr | ChEBI | | Threonin | HMDB | | THREONINE | ChEBI |
|
|---|
| Chemical Formula | C4H9NO3 |
|---|
| IUPAC name | (2S,3R)-2-amino-3-hydroxybutanoic acid |
|---|
| InChI Identifier | InChI=1S/C4H9NO3/c1-2(6)3(5)4(7)8/h2-3,6H,5H2,1H3,(H,7,8)/t2-,3+/m1/s1 |
|---|
| InChI Key | AYFVYJQAPQTCCC-GBXIJSLDSA-N |
|---|
| Isomeric SMILES | C[C@@H](O)[C@H](N)C(O)=O |
|---|
| Average Molecular Weight | 119.1192 |
|---|
| Monoisotopic Molecular Weight | 119.058243159 |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as l-alpha-amino acids. These are alpha amino acids which have the L-configuration of the alpha-carbon atom. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
| Direct Parent | L-alpha-amino acids |
|---|
| Alternative Parents | |
|---|
| Substituents | - L-alpha-amino acid
- Beta-hydroxy acid
- Short-chain hydroxy acid
- Hydroxy acid
- Fatty acid
- Amino acid
- Secondary alcohol
- Carboxylic acid
- Monocarboxylic acid or derivatives
- Alcohol
- Hydrocarbon derivative
- Primary amine
- Organooxygen compound
- Organonitrogen compound
- Organic oxide
- Primary aliphatic amine
- Organopnictogen compound
- Organic oxygen compound
- Carbonyl group
- Organic nitrogen compound
- Amine
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Physico-Chemical Properties - Experimental |
|---|
| Property | Value | Reference |
|---|
| Experimental logP | -2.94 | HANSCH,C ET AL. (1995) |
|---|
| Experimental Water Solubility | 97 mg/mL at 25 oC | YALKOWSKY,SH & DANNENFELSER,RM (1992) |
|---|
| Melting Point | Mp 251-253° dec. | DFC |
|---|
|
| Foods of Origin |
|---|
| Food | Content Range | Average | Reference |
|---|
| Food | | | Reference |
|---|
|
| Production Data |
|---|
| Production Method | commercial |
|---|
| Production Method Reference | Not Available |
|---|
| Production Method Reference File | Not Available |
|---|
| Quantity Available | Production upon request, up to 500 mg |
|---|
| Delivery Time | Not Available |
|---|
| Storage Form | solid |
|---|
| Storage Conditions | -80°C |
|---|
| Stability | Not Available |
|---|
| Purity | Not Available |
|---|
| Spectra |
|---|
| Spectral Data Upon Request | Not Available |
|---|
| Provider Information |
|---|
|
| Commercial Vendors |
|---|
| AKSci | G142 |
|---|
| Glentham | GM4509 |
|---|
| Glentham | GM4012 |
|---|
| MetaSci | HMDB0000167 |
|---|
| Sigma-Aldrich | HMDB0000167 |
|---|
| Toronto Research Chemicals | T405500 |
|---|