| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation date | 2015-10-09 22:28:31 UTC |
|---|
| Update date | 2017-01-19 02:36:13 UTC |
|---|
| FoodComEx ID | PC000111 |
|---|
| FoodDB Record | FDB000484 |
|---|
| Chemical Information |
|---|
| Name | Glycine |
|---|
| Description | Glycine, also known as Gly or aminoacetic acid, belongs to the class of organic compounds known as alpha amino acids. These are amino acids in which the amino group is attached to the carbon atom immediately adjacent to the carboxylate group (alpha carbon). Glycine is a very strong basic compound (based on its pKa). Glycine exists in all living species, ranging from bacteria to humans. Glycine is an odorless tasting compound. Outside of the human body, Glycine is found, on average, in the highest concentration within a few different foods, such as gelatins, gelatin desserts, and beluga whales and in a lower concentration in hedge mustards, pumpkinseed sunfish, and prunus (cherry, plum). Glycine has also been detected, but not quantified in, several different foods, such as boysenberries, fruit juices, giant butterburs, ginsengs, and Chinese mustards. Glycine is most important and simple, nonessential amino acid in humans, animals, and many mammals. Generally, glycine is synthesized from choline, serine, hydroxyproline, and threonine through interorgan metabolism in which kidneys and liver are the primarily involved. Glycine acts as precursor for several key metabolites of low molecular weight such as creatine, glutathione, haem, purines, and porphyrins. There are a number of reports supporting the role of supplementary glycine in prevention of many diseases and disorders including cancer. Dietary supplementation of glycine is effectual has been shown to be effective in treating metabolic disorders in patients with cardiovascular diseases ( PMID: 25332814), several inflammatory diseases, obesity, cancers, and diabetes ( PMID: 27292783). Glycine has also been shown to enhance the quality of sleep and neurological functions. (PMID: 28337245) |
|---|
| CAS Number | 56-40-6 |
|---|
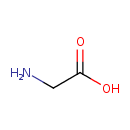
| Structure | |
|---|
| Synonyms | | Synonym | Source |
|---|
| 2-Aminoacetate | biospider | | 2-Aminoacetic acid | biospider | | Aciport | biospider | | Adenine | biospider | | Amino-acetate | biospider | | Amino-acetic acid | biospider | | Aminoacetate | biospider | | Aminoacetic acid | ChEBI | | Aminoacetic acid, 9CI | db_source | | Aminoessigsaeure | ChEBI | | Aminoethanoate | biospider | | Aminoethanoic acid | biospider | | Amitone | biospider | | Athenon | biospider | | E640 | db_source | | FEMA 3287 | db_source | | G | ChEBI | | Glicoamin | biospider | | Glue sugar | db_source | | Gly | db_source | | Glycin | biospider | | Glycocoll | db_source | | Glycolixir | HMDB | | Glycosthene | HMDB | | Glykokoll | ChEBI | | Glyzin | ChEBI | | Gyn-hydralin | HMDB | | H2N-CH2-COOH | ChEBI | | Hgly | ChEBI | | Leimzucker | ChEBI | | Padil | biospider |
|
|---|
| Chemical Formula | C2H5NO2 |
|---|
| IUPAC name | 2-aminoacetic acid |
|---|
| InChI Identifier | InChI=1S/C2H5NO2/c3-1-2(4)5/h1,3H2,(H,4,5) |
|---|
| InChI Key | DHMQDGOQFOQNFH-UHFFFAOYSA-N |
|---|
| Isomeric SMILES | NCC(O)=O |
|---|
| Average Molecular Weight | 75.0666 |
|---|
| Monoisotopic Molecular Weight | 75.032028409 |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as alpha amino acids. These are amino acids in which the amino group is attached to the carbon atom immediately adjacent to the carboxylate group (alpha carbon). |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
| Direct Parent | Alpha amino acids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Alpha-amino acid
- Amino acid
- Carboxylic acid
- Monocarboxylic acid or derivatives
- Organic nitrogen compound
- Organic oxide
- Hydrocarbon derivative
- Primary amine
- Organooxygen compound
- Organonitrogen compound
- Organopnictogen compound
- Primary aliphatic amine
- Organic oxygen compound
- Carbonyl group
- Amine
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Physico-Chemical Properties - Experimental |
|---|
| Property | Value | Reference |
|---|
| Experimental logP | -3.21 | HANSCH,C ET AL. (1995) |
|---|
| Experimental Water Solubility | 249 mg/mL at 25 oC | YALKOWSKY,SH & DANNENFELSER,RM (1992) |
|---|
| Melting Point | Mp 292 (262°)° dec. | DFC |
|---|
|
| Foods of Origin |
|---|
| Food | Content Range | Average | Reference |
|---|
| Food | | | Reference |
|---|
|
| Production Data |
|---|
| Production Method | commercial |
|---|
| Production Method Reference | Not Available |
|---|
| Production Method Reference File | Not Available |
|---|
| Quantity Available | Production upon request, up to 2 g |
|---|
| Delivery Time | Not Available |
|---|
| Storage Form | solid |
|---|
| Storage Conditions | -80°C |
|---|
| Stability | Not Available |
|---|
| Purity | Not Available |
|---|
| Spectra |
|---|
| Spectral Data Upon Request | Not Available |
|---|
| Provider Information |
|---|
|
| Commercial Vendors |
|---|
| AKSci | A486 |
|---|
| Glentham | GM2385 |
|---|
| Glentham | GM2320 |
|---|
| MetaSci | HMDB0000123 |
|---|
| Sigma-Aldrich | HMDB0000123 |
|---|
| Toronto Research Chemicals | G615990 |
|---|