| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation date | 2015-10-09 22:28:25 UTC |
|---|
| Update date | 2017-01-19 02:36:13 UTC |
|---|
| FoodComEx ID | PC000106 |
|---|
| FoodDB Record | FDB011676 |
|---|
| Chemical Information |
|---|
| Name | D-Glucitol |
|---|
| Description | Sorbitol, also known as D-glucitol or L-gulitol, belongs to the class of organic compounds known as sugar alcohols. These are hydrogenated forms of sugars in which the carbonyl group (aldehyde or ketone from the reducing sugar) has been reduced to a primary or secondary hydroxyl group. Sorbitol is a polyhydric alcohol with about half the sweetness of sucrose. Sorbitol occurs naturally in many plants and plant products and is also produced synthetically from glucose. As an industrial chemical, sorbitol is used in the manufacturing of sorbose, propylene glycol and ascorbic acid. It is also used as a plasticizer and stabilizer for vinyl resins, urethane resins and for other rigid foams. Sorbitol also has some pharmaceutical utility. It was formerly used as a diuretic and may still be used as a laxative and in irrigating solutions for some surgical procedures. Sorbitol is also used as a pharmaceutical or cosmetic aid. Sorbitol is used in solution form for moisture-conditioning of cosmetic creams and lotions, toothpaste, gelatins and liquid pharmaceuticals. As a food additive sorbitol functions as a sweetener, humectant, emulsifier, thickener and anticaking agent. Sorbitol is also used as a softener for candy as it acts as a sugar crystallization inhibitor (Hawley's Condensed Chemical Dictionary). As a research chemical, sorbitol is used in photometric determination of Ru (VI) and Ru(VIII) and in acid-base titration of borate (Dictionary of Organic Compounds). Sorbitol occurs widely in nature and is found in simple plants such as algae to higher order plants. Fruits of the plant family Rosacea, which include apples, pears, cherries, apricots, contain appreciable amounts of sorbitol. Particularly rich sources of sorbitol are the fruits of the Sorbus and Crataegus species. |
|---|
| CAS Number | 50-70-4 |
|---|
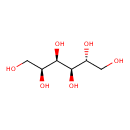
| Structure | |
|---|
| Synonyms | | Synonym | Source |
|---|
| (-)-sorbitol | biospider | | (2R,3R,4R,5S)-hexane-1,2,3,4,5,6-hexol | biospider | | D-(-)-Sorbitol | ChEBI | | D-Glucitol | db_source | | D-Sorbit | ChEBI | | D-SORBITOL | ChEBI | | D-Sorbol | HMDB | | Diakarmon | HMDB | | e 420 | ChEBI | | e-420 | ChEBI | | e420 | ChEBI | | Esasorb | HMDB | | FEMA 3029 | db_source | | Foodol D 70 | HMDB | | g-Ol | ChEBI | | Glc-ol | ChEBI | | Glucarine | biospider | | Glucitol | HMDB | | Karion | HMDB | | Karion instant | HMDB | | Kyowa powder 50m | HMDB | | L-Gulitol | db_source | | Multitol | HMDB | | Neosorb | HMDB | | Neosorb 20/60dc | HMDB | | Neosorb 70/02 | HMDB | | Neosorb 70/70 | HMDB | | Neosorb P 20/60 | HMDB | | Neosorb P 60 | HMDB | | Neosorb P 60W | HMDB | | Nivitin | HMDB | | Resulax | HMDB | | Sionit | HMDB | | Sionit k | HMDB | | Sionite | HMDB | | Sionon | HMDB | | Siosan | HMDB | | Sorbex m | HMDB | | Sorbex R | HMDB | | Sorbex rp | HMDB | | Sorbex S | HMDB | | Sorbex X | HMDB | | Sorbilande | HMDB | | Sorbilax | HMDB | | Sorbit | HMDB | | Sorbit D 70 | HMDB | | Sorbit D-powder | HMDB | | Sorbit dp | HMDB | | Sorbit dp 50 | HMDB | | Sorbit kyowa powder 50m | HMDB | | Sorbit L 70 | HMDB | | Sorbit S | HMDB | | Sorbit t 70 | HMDB | | Sorbit W 70 | HMDB | | Sorbit W-powder | HMDB | | Sorbit W-powder 50 | HMDB | | Sorbit WP | HMDB | | Sorbite | HMDB | | Sorbitol F | HMDB | | Sorbitol fk | HMDB | | Sorbitol FP | HMDB | | Sorbitol S | HMDB | | Sorbitol syrup C | HMDB | | Sorbitur | HMDB | | Sorbo | HMDB | | Sorbogem 712 | HMDB | | Sorbol | HMDB | | Sorbostyl | HMDB |
|
|---|
| Chemical Formula | C6H14O6 |
|---|
| IUPAC name | (2R,3R,4R,5S)-hexane-1,2,3,4,5,6-hexol |
|---|
| InChI Identifier | InChI=1S/C6H14O6/c7-1-3(9)5(11)6(12)4(10)2-8/h3-12H,1-2H2/t3-,4+,5-,6-/m1/s1 |
|---|
| InChI Key | FBPFZTCFMRRESA-JGWLITMVSA-N |
|---|
| Isomeric SMILES | OC[C@H](O)[C@@H](O)[C@H](O)[C@H](O)CO |
|---|
| Average Molecular Weight | 182.1718 |
|---|
| Monoisotopic Molecular Weight | 182.07903818 |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as sugar alcohols. These are hydrogenated forms of carbohydrate in which the carbonyl group (aldehyde or ketone, reducing sugar) has been reduced to a primary or secondary hydroxyl group. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic oxygen compounds |
|---|
| Class | Organooxygen compounds |
|---|
| Sub Class | Carbohydrates and carbohydrate conjugates |
|---|
| Direct Parent | Sugar alcohols |
|---|
| Alternative Parents | |
|---|
| Substituents | - Sugar alcohol
- Monosaccharide
- Secondary alcohol
- Polyol
- Hydrocarbon derivative
- Primary alcohol
- Alcohol
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Physico-Chemical Properties - Experimental |
|---|
| Property | Value | Reference |
|---|
| Experimental logP | -2.20 | SANGSTER (1994) |
|---|
| Experimental Water Solubility | 2750 mg/mL at 30 oC | MULLIN,JW (1972) |
|---|
| Melting Point | Mp 110-112° (anhyd.) | DFC |
|---|
|
| Foods of Origin |
|---|
| Food | Content Range | Average | Reference |
|---|
| Food | | | Reference |
|---|
|
| Production Data |
|---|
| Production Method | commercial |
|---|
| Production Method Reference | Not Available |
|---|
| Production Method Reference File | Not Available |
|---|
| Quantity Available | Production upon request, up to 1 g |
|---|
| Delivery Time | Not Available |
|---|
| Storage Form | solid |
|---|
| Storage Conditions | -80°C |
|---|
| Stability | Not Available |
|---|
| Purity | Not Available |
|---|
| Spectra |
|---|
| Spectral Data Upon Request | Not Available |
|---|
| Provider Information |
|---|
|
| Commercial Vendors |
|---|
| AKSci | 7565AF |
|---|
| AKSci | F763 |
|---|
| Glentham | GC2278 |
|---|
| MetaSci | HMDB0000247 |
|---|
| Sigma-Aldrich | HMDB0000247 |
|---|
| Toronto Research Chemicals | S677100 |
|---|