| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation date | 2015-10-09 22:28:12 UTC |
|---|
| Update date | 2017-01-19 02:36:12 UTC |
|---|
| FoodComEx ID | PC000093 |
|---|
| FoodDB Record | FDB011856 |
|---|
| Chemical Information |
|---|
| Name | L-Histidine |
|---|
| Description | Flavouring ingredient; dietary supplement, nutrient
Histidine (abbreviated as His or H) is one of the 20 standard amino acids present in proteins. Nutritionally, histidine is considered an essential amino acid in human infants. After reaching several years of age, humans begin to synthesize it and it thus becomes a non-essential amino acid. Its codons are CAU and CAC.; Is found abundantly in hemoglobin; The amino acid is a precursor for histamine and carnosine biosynthesis.; The imidazole sidechain of histidine is a common coordinating ligand in metalloproteins and is a part of catalytic sites in certain enzymes. In catalytic triads, the basic nitrogen of histidine is used to abstract a proton from serine, threonine or cysteine to activate it as a nucleophile. In a histidine proton shuttle, histidine is used to quickly shuttle protons, it can do this by abstracting a proton with its basic nitrogen to make a positively-charged intermediate and then use another molecule, a buffer, to extract the proton from its acidic nitrogen. In carbonic anhydrases, a histidine proton shuttle is utilized to rapidly shuttle protons away from a zinc-bound water molecule to quickly regenerate the active form of the enzyme.; histidine is an essential amino acid for infants but not adults. Infants four to six months old require 33 mg/kg of histidine. It is not clear how adults make small amounts of histidine, and dietary sources probably account for most of the histidine in the body. Inborn errors of histidine metabolism exist and are marked by increased histidine levels in the blood. Elevated blood histidine is accompanied by a wide range of symptoms, from mental and physical retardation to poor intellectual functioning, emotional instability, tremor, ataxia and psychosis. histidine in medical therapies has its most promising trials in rheumatoid arthritis where up to 4.5 g daily have been used effectively in severely affected patients. Arthritis patients have been found to have low serum histidine levels, apparently because of too-rapid removal of histidine from their blood. histidine and other imidazole compounds have anti-inflammatory properties. histidine may accomplish this function through a complex interaction with threonine or cysteine and possibly copper. However, copper is usually elevated in rheumatoid arthritis patients and worsens the disease. Other patients besides arthritis patients that have been found to be low in serum histidine are those with chronic renal failure. histidine has been claimed to have been useful in hypertension because of its vasodilatory effects. Claims of its use to improve libido and counteract allergy are without proof at present. histidine may have many other possible functions because it is the precursor of the ubiquitous neurohormone-neurotransmitter histamine. histidine increases histamine in the blood and probably in the brain. Low blood histamine with low serum histidine occurs in rheumatoid arthritis patients. Low blood histamine also occurs in some manic, schizophrenic, high copper and hyperactive groups of psychiatric patients. histidine is a useful therapy in all low histamine patients. ( http://www.dcnutrition.com ). L-Histidine is found in many foods, some of which are lemon verbena, sparkleberry, rowanberry, and walleye. |
|---|
| CAS Number | 71-00-1 |
|---|
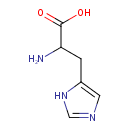
| Structure | |
|---|
| Synonyms | | Synonym | Source |
|---|
| (2S)-2-amino-3-(1H-imidazol-4-yl)propanoic acid | biospider | | (2S)-2-amino-3-(3H-imidazol-4-yl)propanoic acid | biospider | | (l)-histidine | biospider | | (S)-1H-Imidazole-4-alanine | biospider | | (S)-2-Amino-3-(4-imidazolyl)propionic acid | biospider | | (S)-2-amino-3-(4-Imidazolyl)propionsaeure | HMDB | | (S)-4-(2-Amino-2-carboxyethyl)imidazole | biospider | | (S)-a-Amino-1H-imidazole-4-propanoate | biospider | | (S)-a-Amino-1H-imidazole-4-propanoic acid | biospider | | (S)-a-amino-1H-Imidazole-4-propionate | Generator | | (S)-a-amino-1H-Imidazole-4-propionic acid | Generator | | (S)-alpha-Amino-1H-imidazole-4-propanoate | biospider | | (S)-alpha-Amino-1H-imidazole-4-propanoic acid | biospider | | (S)-alpha-Amino-1H-imidazole-4-propionate | biospider | | (S)-alpha-Amino-1H-imidazole-4-propionic acid | biospider | | (s)-histidine | biospider | | (S)-α-amino-1H-imidazole-4-propanoate | Generator | | (S)-α-amino-1H-imidazole-4-propanoic acid | Generator | | (S)-α-amino-1H-imidazole-4-propionate | Generator | | (S)-α-amino-1H-imidazole-4-propionic acid | Generator | | (S)1H-Imidazole-4-alanine | biospider | | 1H-Imidazole-4-alanine, (S)- | biospider | | 1H-Imidazole-4-propanoic acid, α-amino-, (S)- | biospider | | 1H-Imidazole-4-propanoic acid, alpha-amino-, (S)- | biospider | | 3-(1H-imidazol-4-yl)-L-Alanine | biospider | | 4-(2-Amino-2-carboxyethyl)imidazole | biospider | | alpha-Amino-1H-imidazole-4-propionic acid, (S)- | biospider | | alpha-Amino-4(or 5)-imidazolepropionic acid | biospider | | amino-1H-imidazole-4-propanoate | biospider | | amino-1H-imidazole-4-propanoic acid | biospider | | amino-4-imidazoleproprionate | biospider | | amino-4-imidazoleproprionic acid | biospider | | FEMA 3694 | db_source | | Glyoxaline-5-alanine | biospider | | H | ChEBI | | His | ChEBI | | HISTIDINE | ChEBI | | Histidine (usp/inn) | biospider | | Histidine (van) | biospider | | Histidine [usan:inn] | biospider | | Histidine, INN, USAN; L-form | db_source | | Histidine, l- | biospider | | L-(-)-histidine | biospider | | L-Alanine, 3-(1H-imidazol-4-yl)- | biospider | | L-beta-(4-Imidazolyl)-alpha-alanin | biospider | | L-beta-(4-Imidazolyl)alanin | biospider | | L-histidin | biospider | | L-Histidine | biospider | | S-histidine | biospider |
|
|---|
| Chemical Formula | C6H9N3O2 |
|---|
| IUPAC name | 2-amino-3-(1H-imidazol-5-yl)propanoic acid |
|---|
| InChI Identifier | InChI=1S/C6H9N3O2/c7-5(6(10)11)1-4-2-8-3-9-4/h2-3,5H,1,7H2,(H,8,9)(H,10,11) |
|---|
| InChI Key | HNDVDQJCIGZPNO-UHFFFAOYSA-N |
|---|
| Isomeric SMILES | NC(CC1=CN=CN1)C(O)=O |
|---|
| Average Molecular Weight | 155.1546 |
|---|
| Monoisotopic Molecular Weight | 155.069476547 |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as histidine and derivatives. Histidine and derivatives are compounds containing cysteine or a derivative thereof resulting from reaction of cysteine at the amino group or the carboxy group, or from the replacement of any hydrogen of glycine by a heteroatom. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
| Direct Parent | Histidine and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Histidine or derivatives
- Alpha-amino acid
- Imidazolyl carboxylic acid derivative
- Aralkylamine
- Azole
- Imidazole
- Heteroaromatic compound
- Amino acid
- Azacycle
- Organoheterocyclic compound
- Monocarboxylic acid or derivatives
- Carboxylic acid
- Organic nitrogen compound
- Primary amine
- Organooxygen compound
- Organonitrogen compound
- Primary aliphatic amine
- Organic oxide
- Organopnictogen compound
- Carbonyl group
- Organic oxygen compound
- Amine
- Hydrocarbon derivative
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Physico-Chemical Properties - Experimental |
|---|
| Property | Value | Reference |
|---|
| Experimental logP | -3.32 | CHMELIK,J ET AL. (1991) |
|---|
| Experimental Water Solubility | 45.6 mg/mL at 25 oC | YALKOWSKY,SH & DANNENFELSER,RM (1992) |
|---|
| Melting Point | Mp 277° dec. | DFC |
|---|
|
| Foods of Origin |
|---|
| Food | Content Range | Average | Reference |
|---|
| Food | | | Reference |
|---|
|
| Production Data |
|---|
| Production Method | commercial |
|---|
| Production Method Reference | Not Available |
|---|
| Production Method Reference File | Not Available |
|---|
| Quantity Available | Production upon request, up to 6 g |
|---|
| Delivery Time | Not Available |
|---|
| Storage Form | solid |
|---|
| Storage Conditions | -80°C |
|---|
| Stability | Not Available |
|---|
| Purity | Not Available |
|---|
| Spectra |
|---|
| Spectral Data Upon Request | Not Available |
|---|
| Provider Information |
|---|
|
| Commercial Vendors |
|---|
| AKSci | K201 |
|---|
| Glentham | GM1595 |
|---|
| Toronto Research Chemicals | H456010 |
|---|