| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation date | 2015-10-09 22:28:10 UTC |
|---|
| Update date | 2017-01-19 02:36:12 UTC |
|---|
| FoodComEx ID | PC000089 |
|---|
| FoodDB Record | Not Available |
|---|
| Chemical Information |
|---|
| Name | riboflavin |
|---|
| Description | Riboflavin, also known as vitamin b2 or lactoflavin, is a member of the class of compounds known as flavins. Flavins are compounds containing a flavin (7,8-dimethyl-benzo[g]pteridine-2,4-dione) moiety, with a structure characterized by an isoalloaxzine tricyclic ring. Riboflavin is practically insoluble (in water) and a very weakly acidic compound (based on its pKa). Riboflavin can be found in a number of food items such as passion fruit, alaska blueberry, garden rhubarb, and bog bilberry, which makes riboflavin a potential biomarker for the consumption of these food products. Riboflavin can be found primarily in most biofluids, including feces, saliva, urine, and cerebrospinal fluid (CSF), as well as throughout most human tissues. Riboflavin exists in all living species, ranging from bacteria to humans. In humans, riboflavin is involved in the riboflavin metabolism. Moreover, riboflavin is found to be associated with alcoholism and anorexia nervosa. Riboflavin is a non-carcinogenic (not listed by IARC) potentially toxic compound. Riboflavin is a drug which is used for the treatment of ariboflavinosis (vitamin b2 deficiency). Riboflavin was discovered in 1920, isolated in 1933, and first made in 1935. It is on the World Health Organization's List of Essential Medicines, the most effective and safe medicines needed in a health system. Riboflavin is available as a generic medication and over the counter. In the United States a month of supplements costs less than 25 USD. Some countries require its addition to grains . Binds to riboflavin hydrogenase, riboflavin kinase, and riboflavin synthase. Riboflavin is the precursor of flavin mononucleotide (FMN, riboflavin monophosphate) and flavin adenine dinucleotide (FAD). The antioxidant activity of riboflavin is principally derived from its role as a precursor of FAD and the role of this cofactor in the production of the antioxidant reduced glutathione. Reduced glutathione is the cofactor of the selenium-containing glutathione peroxidases among other things. The glutathione peroxidases are major antioxidant enzymes. Reduced glutathione is generated by the FAD-containing enzyme glutathione reductase (DrugBank). Binds to riboflavin hydrogenase, riboflavin kinase, and riboflavin synthase. Riboflavin is the precursor of flavin mononucleotide (FMN, riboflavin monophosphate) and flavin adenine dinucleotide (FAD). The antioxidant activity of riboflavin is principally derived from its role as a precursor of FAD and the role of this cofactor in the production of the antioxidant reduced glutathione. Reduced glutathione is the cofactor of the selenium-containing glutathione peroxidases among other things. The glutathione peroxidases are major antioxidant enzymes. Reduced glutathione is generated by the FAD-containing enzyme glutathione reductase (T3DB). |
|---|
| CAS Number | Not Available |
|---|
| Structure | |
|---|
| Synonyms | |
|---|
| Chemical Formula | Not Available |
|---|
| IUPAC name | Not Available |
|---|
| InChI Identifier | InChI=1S/C17H20N4O6/c1-7-3-9-10(4-8(7)2)21(5-11(23)14(25)12(24)6-22)15-13(18-9)16(26)20-17(27)19-15/h3-4,11-12,14,22-25H,5-6H2,1-2H3,(H,20,26,27)/t11-,12+,14-/m0/s1 |
|---|
| InChI Key | AUNGANRZJHBGPY-SCRDCRAPSA-N |
|---|
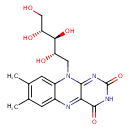
| Isomeric SMILES | CC1=C(C)C=C2N(C[C@H](O)[C@H](O)[C@H](O)CO)C3=NC(=O)NC(=O)C3=NC2=C1 |
|---|
| Average Molecular Weight | 376.3639 |
|---|
| Monoisotopic Molecular Weight | 376.138284392 |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as flavins. Flavins are compounds containing a flavin (7,8-dimethyl-benzo[g]pteridine-2,4-dione) moiety, with a structure characterized by an isoalloaxzine tricyclic ring. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Pteridines and derivatives |
|---|
| Sub Class | Alloxazines and isoalloxazines |
|---|
| Direct Parent | Flavins |
|---|
| Alternative Parents | |
|---|
| Substituents | - Flavin
- Diazanaphthalene
- Quinoxaline
- Pyrimidone
- Pyrazine
- Pyrimidine
- Benzenoid
- Heteroaromatic compound
- Vinylogous amide
- Secondary alcohol
- Lactam
- Polyol
- Azacycle
- Alcohol
- Hydrocarbon derivative
- Organic oxide
- Organopnictogen compound
- Organic oxygen compound
- Primary alcohol
- Organooxygen compound
- Organonitrogen compound
- Organic nitrogen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Physico-Chemical Properties - Experimental |
|---|
| Property | Value | Reference |
|---|
| Experimental logP | Not Available | |
|---|
| Experimental Water Solubility | Not Available | |
|---|
| Melting Point | Not Available | |
|---|
|
| Foods of Origin |
|---|
| Food | Content Range | Average | Reference |
|---|
| Food | | | Reference |
|---|
|
| Production Data |
|---|
| Production Method | commercial |
|---|
| Production Method Reference | Not Available |
|---|
| Production Method Reference File | Not Available |
|---|
| Quantity Available | Production upon request |
|---|
| Delivery Time | Not Available |
|---|
| Storage Form | solid |
|---|
| Storage Conditions | -80°C |
|---|
| Stability | Not Available |
|---|
| Purity | Not Available |
|---|
| Spectra |
|---|
| Spectral Data Upon Request | Not Available |
|---|
| Provider Information |
|---|
|
| Commercial Vendors |
|---|
| Not Available |