| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation date | 2015-10-09 22:28:08 UTC |
|---|
| Update date | 2017-01-19 02:36:12 UTC |
|---|
| FoodComEx ID | PC000087 |
|---|
| FoodDB Record | FDB000921 |
|---|
| Chemical Information |
|---|
| Name | Urocanic acid |
|---|
| Description | Urocanic acid is an intermediate in the catabolism of L-histidine.; Urocanic is a breakdown (deamination) product of histidine. In the liver, urocanic acid is an intermediate in the conversion of histidine to glutamic acid, whereas in the epidermis, it accumulates and may be both a UV protectant and an immunoregulator. Urocanic acid (UA) exists as a trans isomer (t-UA, approximately 30 mg/cm2) in the uppermost layer of the skin (stratum corneum). t-UA is formed as the cells of the second layer of skin become metabolically inactive. During this process, proteins and membranes degrade, histidine is released, and histidase (histidine ammonia lyase) catalyzes the deamination of histidine to form t-UA. t-UA accumulates in the epidermis until removal by either the monthly skin renewal cycle or sweat. Upon absorption of UV light, the naturally occurring t-UA isomerizes to its cis form, c-UA. Because DNA lesions (e.g., pyrimidine dimers) in the lower epidermis can result from UV-B absorption, initial research proposed that t-UA acted as a natural sunscreen absorbing UV-B in the stratum corneum before the damaging rays could penetrate into lower epidermal zones. Researchers have found that c-UA also suppresses contact hypersensitivity and delayed hypersensitivity, reduces the Langerhans cell count in the epidermis, prolongs skin-graft survival time, and affects natural killer cell activity. Urocanic acid is found in mung bean. |
|---|
| CAS Number | 104-98-3 |
|---|
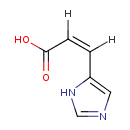
| Structure | |
|---|
| Synonyms | | Synonym | Source |
|---|
| (2e)-3-(1H-Imidazol-4-yl)acrylate | Generator | | (e)-3-(1H-Imidazol-4-yl)-2-propenoate | Generator | | 3-(1H-Imidazol-4-yl)-2-propenoate | biospider | | 3-(1H-Imidazol-4-yl)-2-propenoic acid | HMDB | | 3-(1H-Imidazol-4-yl)-2-propenoic acid, 9CI | db_source | | 3-(1H-Imidazol-4-yl)acrylate | biospider | | 3-(1H-Imidazol-4-yl)acrylic acid | biospider | | 3-(4-Imidazolyl)acrylate | biospider | | 3-(4-Imidazolyl)acrylic acid | HMDB | | 4-Imidazoleacrylic acid, 8CI | db_source | | 5-Imidazoleacrylate | HMDB | | 5-Imidazoleacrylate;imidazoleacrylic acid | biospider | | 5-Imidazoleacrylic acid | HMDB | | Imidazole-4-acrylate | biospider | | Imidazole-4-acrylic acid | HMDB | | Imidazoleacrylic acid | HMDB | | Trans-urocanate | biospider | | Urocanate | Generator | | Urocaninic acid | db_source |
|
|---|
| Chemical Formula | C6H6N2O2 |
|---|
| IUPAC name | (2Z)-3-(1H-imidazol-5-yl)prop-2-enoic acid |
|---|
| InChI Identifier | InChI=1S/C6H6N2O2/c9-6(10)2-1-5-3-7-4-8-5/h1-4H,(H,7,8)(H,9,10)/b2-1- |
|---|
| InChI Key | LOIYMIARKYCTBW-UPHRSURJSA-N |
|---|
| Isomeric SMILES | OC(=O)\C=C/C1=CN=CN1 |
|---|
| Average Molecular Weight | 138.124 |
|---|
| Monoisotopic Molecular Weight | 138.042927446 |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as imidazolyl carboxylic acids and derivatives. These are organic compounds containing a carboxylic acid chain (of at least 2 carbon atoms) linked to an imidazole ring. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Azoles |
|---|
| Sub Class | Imidazoles |
|---|
| Direct Parent | Imidazolyl carboxylic acids and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Imidazolyl carboxylic acid derivative
- Heteroaromatic compound
- Azacycle
- Monocarboxylic acid or derivatives
- Carboxylic acid
- Carboxylic acid derivative
- Organic nitrogen compound
- Organic oxygen compound
- Organopnictogen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Organonitrogen compound
- Carbonyl group
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Physico-Chemical Properties - Experimental |
|---|
| Property | Value | Reference |
|---|
| Experimental logP | Not Available | |
|---|
| Experimental Water Solubility | 1.5 mg/mL at 17 oC | YALKOWSKY,SH & DANNENFELSER,RM (1992) |
|---|
| Melting Point | Mp 243-245° | DFC |
|---|
|
| Foods of Origin |
|---|
| Food | Content Range | Average | Reference |
|---|
| Food | | | Reference |
|---|
|
| Production Data |
|---|
| Production Method | commercial |
|---|
| Production Method Reference | Not Available |
|---|
| Production Method Reference File | Not Available |
|---|
| Quantity Available | Production upon request, up to 1 g |
|---|
| Delivery Time | Not Available |
|---|
| Storage Form | solid |
|---|
| Storage Conditions | -80°C |
|---|
| Stability | Not Available |
|---|
| Purity | Not Available |
|---|
| Spectra |
|---|
| Spectral Data Upon Request | Not Available |
|---|
| Provider Information |
|---|
|
| Commercial Vendors |
|---|
| AKSci | 1164AA |
|---|