| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation date | 2015-10-09 22:28:05 UTC |
|---|
| Update date | 2017-01-19 02:36:12 UTC |
|---|
| FoodComEx ID | PC000083 |
|---|
| FoodDB Record | FDB000787 |
|---|
| Chemical Information |
|---|
| Name | L-Asparagine |
|---|
| Description | L-Asparagine, also known as Asn or aspartamic acid, belongs to the class of organic compounds known as asparagine and derivatives. Asparagine and derivatives are compounds containing asparagine or a derivative thereof resulting from reaction of asparagine at the amino group or the carboxy group, or from the replacement of any hydrogen of glycine by a heteroatom. L-Asparagine is a drug which is used for nutritional supplementation, also for treating dietary shortage or imbalance. L-Asparagine is a very strong basic compound (based on its pKa). L-Asparagine exists in all living species, ranging from bacteria to humans. Within humans, L-asparagine participates in a number of enzymatic reactions. In particular, L-asparagine and L-glutamic acid can be biosynthesized from L-aspartic acid and L-glutamine through the action of the enzyme asparagine synthetase [glutamine-hydrolyzing]. In addition, L-asparagine can be converted into L-aspartic acid through the action of the enzyme isoaspartyl peptidase/l-asparaginase. In humans, L-asparagine is involved in aspartate metabolism. L-Asparagine is an odorless tasting compound. Outside of the human body, L-Asparagine is found, on average, in the highest concentration within a few different foods, such as white lupines, wheats, and oats and in a lower concentration in sacred lotus, parsnips, and pineapples. L-Asparagine has also been detected, but not quantified in, several different foods, such as colorado pinyons, opium poppies, lentils, green beans, and watermelons. This could make L-asparagine a potential biomarker for the consumption of these foods. L-Asparagine is a potentially toxic compound. An optically active form of asparagine having L-configuration. |
|---|
| CAS Number | 70-47-3 |
|---|
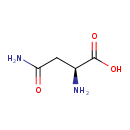
| Structure | |
|---|
| Synonyms | | Synonym | Source |
|---|
| (-)-Asparagine | biospider | | (2S)-2-amino-3-Carbamoylpropanoate | Generator | | (2S)-2-Amino-3-carbamoylpropanoic acid | biospider | | (2S)-2,4-Diamino-4-oxobutanoate | Generator | | (2S)-2,4-Diamino-4-oxobutanoic acid | biospider | | (S)-2-amino-3-Carbamoylpropanoate | Generator | | (S)-2-Amino-3-carbamoylpropanoic acid | biospider | | (S)-2-Aminosuccinic acid 4-amide | biospider | | (S)-2,4-Diamino-4-oxobutanoate | biospider | | (S)-2,4-Diamino-4-oxobutanoic acid | biospider | | (S)-Asparagine | biospider | | 2-Aminosuccinamate | Generator | | 2-Aminosuccinamic acid | ChEBI | | 2-Aminosuccinamic acid, L- | biospider | | a-Aminosuccinamate | Generator | | a-Aminosuccinamic acid | Generator | | Agedoite | HMDB | | alpha Amminosuccinamate | HMDB | | alpha Amminosuccinamic acid | HMDB | | alpha-Aminosuccinamate | Generator | | alpha-Aminosuccinamic acid | ChEBI | | Altheine | HMDB | | Asn | ChEBI | | ASPARAGINE | ChEBI | | Asparagine acid | HMDB | | Asparagine, 9CI; L-form | db_source | | Asparagine, L- (8CI) | biospider | | Asparamide | HMDB | | Aspartamate | Generator | | Aspartamic acid | ChEBI | | Aspartic acid amide | HMDB | | Aspartic acid b-amide | HMDB | | Aspartic acid beta amide | HMDB | | b2,4-(S)-Diamino-4-oxo-utanoate | HMDB | | b2,4-(S)-Diamino-4-oxo-utanoic acid | HMDB | | Crystal VI | HMDB | | L-2-Aminosuccinamate | Generator | | L-2-Aminosuccinamic acid | biospider | | L-2,4-Diamino-4-oxobutanoate | biospider | | L-2,4-Diamino-4-oxobutanoic acid | biospider | | L-Asparagin | ChEBI | | L-Asparagine (9CI) | biospider | | L-Asparatamine | biospider | | L-Aspartamine | biospider | | L-Aspartate b-amide | Generator | | L-Aspartate beta-amide | Generator | | L-Aspartate β-amide | Generator | | L-Aspartic acid 4-amide | biospider | | L-Aspartic acid b-amide | Generator | | L-Aspartic acid beta-amide | biospider | | L-Aspartic acid β-amide | Generator | | L-b-Asparagine | biospider | | L-beta-Asparagine | biospider | | N | ChEBI | | α-aminosuccinamate | Generator | | α-aminosuccinamic acid | Generator |
|
|---|
| Chemical Formula | C4H8N2O3 |
|---|
| IUPAC name | (2S)-2-amino-3-carbamoylpropanoic acid |
|---|
| InChI Identifier | InChI=1S/C4H8N2O3/c5-2(4(8)9)1-3(6)7/h2H,1,5H2,(H2,6,7)(H,8,9)/t2-/m0/s1 |
|---|
| InChI Key | DCXYFEDJOCDNAF-REOHCLBHSA-N |
|---|
| Isomeric SMILES | N[C@@H](CC(N)=O)C(O)=O |
|---|
| Average Molecular Weight | 132.1179 |
|---|
| Monoisotopic Molecular Weight | 132.053492132 |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as asparagine and derivatives. Asparagine and derivatives are compounds containing asparagine or a derivative thereof resulting from reaction of asparagine at the amino group or the carboxy group, or from the replacement of any hydrogen of glycine by a heteroatom. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
| Direct Parent | Asparagine and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Asparagine or derivatives
- Alpha-amino acid
- L-alpha-amino acid
- Fatty amide
- Fatty acyl
- Fatty acid
- Carboxamide group
- Amino acid
- Primary carboxylic acid amide
- Carboxylic acid
- Monocarboxylic acid or derivatives
- Organic nitrogen compound
- Primary amine
- Organooxygen compound
- Organonitrogen compound
- Hydrocarbon derivative
- Primary aliphatic amine
- Organic oxide
- Organopnictogen compound
- Organic oxygen compound
- Carbonyl group
- Amine
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Physico-Chemical Properties - Experimental |
|---|
| Property | Value | Reference |
|---|
| Experimental logP | -3.82 | CHMELIK,J ET AL. (1991) |
|---|
| Experimental Water Solubility | 29.4 mg/mL at 25 oC | YALKOWSKY,SH & DANNENFELSER,RM (1992) |
|---|
| Melting Point | Mp 226-227 dec. (slow heat) | DFC |
|---|
|
| Foods of Origin |
|---|
| Food | Content Range | Average | Reference |
|---|
| Food | | | Reference |
|---|
|
| Production Data |
|---|
| Production Method | commercial |
|---|
| Production Method Reference | Not Available |
|---|
| Production Method Reference File | Not Available |
|---|
| Quantity Available | Production upon request, up to 2 g |
|---|
| Delivery Time | Not Available |
|---|
| Storage Form | solid |
|---|
| Storage Conditions | -80°C |
|---|
| Stability | Not Available |
|---|
| Purity | Not Available |
|---|
| Spectra |
|---|
| Spectral Data Upon Request | Not Available |
|---|
| Provider Information |
|---|
|
| Commercial Vendors |
|---|
| AKSci | K189 |
|---|
| Glentham | GM9590 |
|---|
| MetaSci | HMDB0000168 |
|---|
| Sigma-Aldrich | HMDB0000168 |
|---|
| Toronto Research Chemicals | A790028 |
|---|