| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation date | 2015-10-09 22:28:04 UTC |
|---|
| Update date | 2017-01-19 02:36:12 UTC |
|---|
| FoodComEx ID | PC000082 |
|---|
| FoodDB Record | FDB000522 |
|---|
| Chemical Information |
|---|
| Name | L-Homoserine |
|---|
| Description | Present in germinating peas, Jack bean seeds (Canavalia ensiformis) and the seedlings of many leguminous plants
Homoserine is a more reactive variant of the amino acid serine. In this variant, the hydroxyl side chain contains an additional CH2 group which brings the hydroxyl group closer to its own carboxyl group, allowing it to chemically react to form a five-membered ring. This occurs at the point that amino acids normally join to their neighbours in a peptide bond.; Homoserine is therefore unsuitable for forming proteins and has been eliminated from the repertoire of amino acids used by living things.; Homoserine is the final product on the C-terminal end of the N-terminal fragment following a cyanogen bromide cleavage. (wikipedia); Homoserine is an intermediate in the biosynthesis of three essential amino acids: methionine, threonine (an isomer of homoserine), and isoleucine. It forms by two reductions of aspartic acid via the intermediacy of aspartate semialdehyde.; Homoserine is an ?-amino acid with the chemical formula HO2CCH(NH2)CH2CH2OH. L-Homoserine is not one of the common amino acids encoded by DNA. It differs from the proteinogenic amino acid serine by insertion of an additional methylene group. Homoserine, or its lactone form, is the product of a cyanogen bromide cleavage of a peptide by degradation of methionine. L-Homoserine is found in many foods, some of which are cornmint, star fruit, common wheat, and nance. |
|---|
| CAS Number | 672-15-1 |
|---|
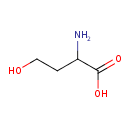
| Structure | |
|---|
| Synonyms | | Synonym | Source |
|---|
| (2S)-2-amino-4-Hydroxybutanoate | Generator | | (2S)-2-amino-4-hydroxybutanoic acid | biospider | | (S)-2-amino-4-hydroxy-Butanoate | biospider | | (S)-2-amino-4-hydroxy-Butanoic acid | biospider | | (S)-2-Amino-4-hydroxybutanoate | biospider | | (S)-2-Amino-4-hydroxybutanoic acid | biospider | | (S)-2-Amino-4-hydroxybutyric acid | biospider | | (s)-homoserine | biospider | | 2-amino-4-hydroxy-Butyrate | biospider | | 2-amino-4-hydroxy-Butyric acid | biospider | | 2-amino-4-hydroxy-L-Butyrate | biospider | | 2-amino-4-hydroxy-L-Butyric acid | biospider | | 2-Amino-4-hydroxybutanoate | biospider | | 2-Amino-4-hydroxybutanoic acid | biospider | | 2-Amino-4-hydroxybutanoic acid; L-form | db_source | | 2-Amino-4-hydroxybutyrate | biospider | | 2-Amino-4-hydroxybutyric acid | biospider | | Butanoic acid, 2-amino-4-hydroxy-, (S)- | biospider | | Butyric acid, 2-amino-4-hydroxy-, L- | biospider | | Butyric acid, 2-amino-4-hydroxy-, L- (8CI) | biospider | | Homoserine | biospider | | Homoserine (van) | biospider | | HSE | biospider | | L-homoserine | biospider |
|
|---|
| Chemical Formula | C4H9NO3 |
|---|
| IUPAC name | 2-amino-4-hydroxybutanoic acid |
|---|
| InChI Identifier | InChI=1S/C4H9NO3/c5-3(1-2-6)4(7)8/h3,6H,1-2,5H2,(H,7,8) |
|---|
| InChI Key | UKAUYVFTDYCKQA-UHFFFAOYSA-N |
|---|
| Isomeric SMILES | NC(CCO)C(O)=O |
|---|
| Average Molecular Weight | 119.1192 |
|---|
| Monoisotopic Molecular Weight | 119.058243159 |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as alpha amino acids. These are amino acids in which the amino group is attached to the carbon atom immediately adjacent to the carboxylate group (alpha carbon). |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
| Direct Parent | Alpha amino acids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Alpha-amino acid
- Short-chain hydroxy acid
- Fatty acid
- 1,3-aminoalcohol
- Amino acid
- Carboxylic acid
- Monocarboxylic acid or derivatives
- Organic nitrogen compound
- Hydrocarbon derivative
- Primary amine
- Primary alcohol
- Organooxygen compound
- Organonitrogen compound
- Organic oxide
- Primary aliphatic amine
- Organopnictogen compound
- Carbonyl group
- Amine
- Organic oxygen compound
- Alcohol
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Physico-Chemical Properties - Experimental |
|---|
| Property | Value | Reference |
|---|
| Experimental logP | Not Available | |
|---|
| Experimental Water Solubility | 1000 mg/mL at 30 oC | BEILSTEIN |
|---|
| Melting Point | Mp 203° dec. | DFC |
|---|
|
| Foods of Origin |
|---|
| Food | Content Range | Average | Reference |
|---|
| Food | | | Reference |
|---|
|
| Production Data |
|---|
| Production Method | commercial |
|---|
| Production Method Reference | Not Available |
|---|
| Production Method Reference File | Not Available |
|---|
| Quantity Available | Production upon request, up to 6 g |
|---|
| Delivery Time | Not Available |
|---|
| Storage Form | solid |
|---|
| Storage Conditions | -80°C |
|---|
| Stability | Not Available |
|---|
| Purity | Not Available |
|---|
| Spectra |
|---|
| Spectral Data Upon Request | Not Available |
|---|
| Provider Information |
|---|
|
| Commercial Vendors |
|---|
| AKSci | J90653 |
|---|
| AKSci | K105 |
|---|
| Alfa Aesar | HMDB0000719 |
|---|
| MetaSci | HMDB0000719 |
|---|
| Toronto Research Chemicals | H615010 |
|---|