| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation date | 2015-10-09 22:28:01 UTC |
|---|
| Update date | 2017-01-19 02:36:12 UTC |
|---|
| FoodComEx ID | PC000075 |
|---|
| FoodDB Record | FDB022083 |
|---|
| Chemical Information |
|---|
| Name | Ribitol |
|---|
| Description | D-Arabitol or D-arabinitol, also known as klinit or lyxitol, belongs to the class of organic compounds known as sugar alcohols, a type of polyols. These are hydrogenated forms of carbohydrates in which the carbonyl group (aldehyde or ketone, reducing sugar) has been reduced to a primary or secondary hydroxyl group. A polyol is an organic compound containing many hydroxyls. D-Arabitol is neutral compound. Polyols, classified by the number of carbon atoms and include the sugar alcohols, are linked to the pentose phosphate pathway (PPP). The PPP generates, NADPH and pentoses (5-carbon sugars) as well as ribose 5-phosphate, a precursor for the synthesis of nucleotides. While the pentose phosphate pathway oxidizes glucose, its primary role is anabolic rather than catabolic. Polyols, including D-arabitol, accumulates in body fluids in individuals with two defects in PPP, involving the enzymes ribose-5-phosphate isomerase (RPI) and transaldolase (PMID: 18987987). A patient with leukoencephalopathy and peripheral neuropathy, with a strong brain-CSF-plasma gradient of C5 polyols such as D-arabitol, was identified as suffering from RPI deficiency. Another patient displaying mainly liver problems and increased concentrations of polyols, mainly D-arabitol, was diagnosed with transaldolase deficiency. However, the mechanism of pathophysiology of polyols in patients with defects in the PPP is poorly understood. It is thought that D-arabitol is a metabolic end-product in humans. The mechanisms of brain and neuronal damage in RPI deficiency may result from polyol accumulation, causing secondary brain dysfunction (PMID: 16435225). D-Arabitol is a product of the enzyme D-arabinitol 4-dehydrogenase (EC 1.1.1.11) in the pentose and glucuronate interconversion pathway. D-Arabitol accumulated in a Penicillium rubens strain deleted of four highly expressed biosynthetic gene clusters that produce penicillin, roquefortine, chrysogine and fungisporin. The transcriptome of the P. rubens strain had changed when the gene cluster was removed, resulting in increased expression of D-arabinitol 4-dehydrogenase (PMID: 32376967). D-Arabitol, a fungal metabolite, is used as a marker for invasive candidiasis or infection by Candida fungal species (PMID: 15183861; PMID: 10647119). It is also a metabolite of Debaryomyces, Pichia and Zygosaccharomyces (PMID: 25809659). |
|---|
| CAS Number | 488-81-3 |
|---|
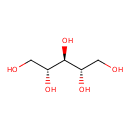
| Structure | |
|---|
| Synonyms | | Synonym | Source |
|---|
| 1,2,3,4,5-pentanepentol | hmdb | | Adonit | hmdb | | Adonite | hmdb | | Adonitol | hmdb | | D-Adonitol | ChEBI | | D-Ribitol | ChEBI | | L-Ribitol | ChEBI | | pentitol | hmdb | | Ribitol | hmdb |
|
|---|
| Chemical Formula | C5H12O5 |
|---|
| IUPAC name | (2R,3s,4S)-pentane-1,2,3,4,5-pentol |
|---|
| InChI Identifier | InChI=1S/C5H12O5/c6-1-3(8)5(10)4(9)2-7/h3-10H,1-2H2/t3-,4+,5- |
|---|
| InChI Key | HEBKCHPVOIAQTA-ZXFHETKHSA-N |
|---|
| Isomeric SMILES | OC[C@H](O)[C@H](O)[C@H](O)CO |
|---|
| Average Molecular Weight | 152.1458 |
|---|
| Monoisotopic Molecular Weight | 152.068473494 |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as sugar alcohols. These are hydrogenated forms of carbohydrate in which the carbonyl group (aldehyde or ketone, reducing sugar) has been reduced to a primary or secondary hydroxyl group. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic oxygen compounds |
|---|
| Class | Organooxygen compounds |
|---|
| Sub Class | Carbohydrates and carbohydrate conjugates |
|---|
| Direct Parent | Sugar alcohols |
|---|
| Alternative Parents | |
|---|
| Substituents | - Sugar alcohol
- Monosaccharide
- Secondary alcohol
- Polyol
- Hydrocarbon derivative
- Primary alcohol
- Alcohol
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Physico-Chemical Properties - Experimental |
|---|
| Property | Value | Reference |
|---|
| Experimental logP | Not Available | |
|---|
| Experimental Water Solubility | Not Available | |
|---|
| Melting Point | Not Available | |
|---|
|
| Foods of Origin |
|---|
| Food | Content Range | Average | Reference |
|---|
| Food | | | Reference |
|---|
|
| Production Data |
|---|
| Production Method | commercial |
|---|
| Production Method Reference | Not Available |
|---|
| Production Method Reference File | Not Available |
|---|
| Quantity Available | Production upon request, up to 2 g |
|---|
| Delivery Time | Not Available |
|---|
| Storage Form | solid |
|---|
| Storage Conditions | -80°C |
|---|
| Stability | Not Available |
|---|
| Purity | Not Available |
|---|
| Spectra |
|---|
| Spectral Data Upon Request | Not Available |
|---|
| Provider Information |
|---|
|
| Commercial Vendors |
|---|
| AKSci | C452 |
|---|
| Glentham | GC5301 |
|---|
| MetaSci | HMDB0000508 |
|---|
| Tokyo Chemical Industry | HMDB0000508 |
|---|