| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation date | 2015-10-09 22:27:54 UTC |
|---|
| Update date | 2017-01-19 02:36:11 UTC |
|---|
| FoodComEx ID | PC000067 |
|---|
| FoodDB Record | Not Available |
|---|
| Chemical Information |
|---|
| Name | L-Phenylalanine |
|---|
| Description | Found in many foods. It is used in cocoa substitutes. Flavouring ingredient; dietary supplement
L-phenylalanine can also be converted into L-tyrosine, another one of the DNA-encoded amino acids. L-tyrosine in turn is converted into L-DOPA, which is further converted into dopamine, norepinephrine (noradrenaline), and epinephrine (adrenaline). The latter three are known as the catecholamines.; Phenylalanine (abbreviated as Phe or F) is an ?-amino acid with the formula HO2CCH(NH2)CH2C6H5, which is found naturally in the breast milk of mammals and manufactured for food and drink products and are also sold as nutritional supplements for their reputed analgesic and antidepressant effects. Phenylalanine is structurally closely related to dopamine, epinephrine (adrenaline) and tyrosine. It is a direct precursor to the neuromodulator phenylethylamine, a commonly used dietary supplement.; Phenylalanine is an essential amino acid and the precursor for the amino acid tyrosine. Like tyrosine, it is the precursor of catecholamines in the body (tyramine, dopamine, epinephrine and norepinephrine). The psychotropic drugs (mescaline, morphine, codeine, and papaverine) also have phenylalanine as a constituent. Phenylalanine is a precursor of the neurotransmitters called catecholamines, which are adrenalin-like substances. Phenylalanine is highly concentrated in the human brain and plasma. Normal metabolism of phenylalanine requires biopterin, iron, niacin, vitamin B6, copper and vitamin C. An average adult ingests 5 g of phenylalanine per day and may optimally need up to 8 g daily. Phenylalanine is highly concentrated in high protein foods, such as meat, cottage cheese and wheat germ. A new dietary source of phenylalanine is artificial sweeteners containing aspartame. Aspartame appears to be nutritious except in hot beverages; This essential amino acid is classified as nonpolar because of the hydrophobic nature of the benzyl side chain. The codons for L-phenylalanine are UUU and UUC. It is a white, powdery solid. L-Phenylalanine (LPA) is an electrically-neutral amino acid, one of the twenty common amino acids used to biochemically form proteins, coded for by DNA.; It is used by the brain to produce Norepinephrine, a chemical that transmits signals between nerve cells and the brain; however, it should be avoided by phenylketonurics and pregnant women. Phenylketonurics, who have a genetic error of phenylalanine metabolism, have elevated serum plasma levels of phenylalanine up to 400 times normal. Mild phenylketonuria can be an unsuspected cause of hyperactivity, learning problems, and other developmental problems in children. Phenylalanine can be an effective pain reliever. Its use in premenstrual syndrome and Parkinson's may enhance the effects of acupuncture and electric transcutaneous nerve stimulation (TENS). Phenylalanine and tyrosine, like L-dopa, produce a catecholamine effect. Phenylalanine is better absorbed than tyrosine and may cause fewer headaches. Low phenylalanine diets have been prescribed for certain cancers with mixed results. Some tumors use more phenylalanine (particularly melatonin-producing tumors called melanoma). One strategy is to exclude this amino acid from the diet, i.e., a Phenylketonuria (PKU) diet (compliance is a difficult issue; it is hard to quantify and is under-researched). The other strategy is just to increase phenylalanine's competing amino acids, i.e., tryptophan, valine, isoleucine and leucine, but not tyrosine. (http://www.dcnutrition.com) |
|---|
| CAS Number | 63-91-2 |
|---|
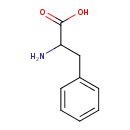
| Structure | |
|---|
| Synonyms | | Synonym | Source |
|---|
| (-)-β-phenylalanine | biospider | | (-)-beta-phenylalanine | biospider | | (2S)-2-amino-3-phenylpropanoic acid | biospider | | (l)-phenylalanine | biospider | | (s)-(-)-phenylalanine | biospider | | (s)-α-aminobenzenepropanoic acid | biospider | | (S)-2-amino-3-phenylpropanoate | biospider | | (S)-2-amino-3-phenylpropanoic acid | biospider | | (S)-2-Amino-3-phenylpropionate | biospider | | (S)-2-Amino-3-phenylpropionic acid | biospider | | (S)-a-amino-b-Phenylpropionate | Generator | | (S)-a-amino-b-Phenylpropionic acid | Generator | | (s)-alpha-amino-benzenepropanoate | biospider | | (s)-alpha-amino-benzenepropanoic acid | biospider | | (s)-alpha-amino-beta-phenylpropionate | biospider | | (s)-alpha-amino-beta-phenylpropionic acid | biospider | | (s)-alpha-aminobenzenepropanoate | biospider | | (s)-alpha-aminobenzenepropanoic acid | biospider | | (s)-alpha-aminohydrocinnamate | biospider | | (s)-alpha-aminohydrocinnamic acid | biospider | | (S)-Phenylalanine | HMDB | | (S)-α-amino-β-phenylpropionate | Generator | | (S)-α-amino-β-phenylpropionic acid | Generator | | α-alanine, l- | biospider | | α-amino-, (s)- | biospider | | α-amino-α-aminohydrocinnamic acid | biospider | | α-amino-β-phenylpropionic acid | biospider | | α-aminohydrocinnamic acid | biospider | | β-phenyl-α-alanine | biospider | | β-phenyl-α-alanine, l- | biospider | | β-phenyl-l-alanine | biospider | | β-phenylalanine | biospider | | 1usi | biospider | | 2-Amino-3-phenylpropionic acid, L- | biospider | | 3-Phenyl-L-alanine | ChEBI | | Alpha-amino-beta-phenylpropionic acid, l- | biospider | | Alpha-aminohydrocinnamate | biospider | | Alpha-aminohydrocinnamic acid | biospider | | Alpha-aminohydrocinnamic acid, l- | biospider | | b-Phenyl-L-alanine | Generator | | Benzenepropanoic acid, α-amino-, (s)- | biospider | | Benzenepropanoic acid, alpha-amino-, (s)- | biospider | | Beta-phenyl-alpha-alanine | biospider | | Beta-phenyl-l-alanine | biospider | | Beta-phenylalanine | biospider | | Beta-phenylalnine, (-)- | biospider | | Endophenyl | biospider | | F | ChEBI | | FEMA 3585 | db_source | | Hydrocinnamic acid, α-amino- | biospider | | Hydrocinnamic acid, alpha-amino- | biospider | | L-α-alanine (van) | biospider | | L-β-phenylalanine | biospider | | L-2-Amino-3-phenylpropionate | biospider | | L-2-Amino-3-phenylpropionic acid | biospider | | L-Alanine, 3-phenyl- | biospider | | L-Phenylalanine (JP15) | biospider | | L-Phenylalanine, JAN | db_source | | L-phenylalinine | biospider | | PAL | biospider | | PHE | biospider | | Phenyl-α-alanine | biospider | | Phenyl-alanine | biospider | | Phenylalamine | HMDB | | PHENYLALANINE | ChEBI | | Phenylalanine (usp/inn) | biospider | | Phenylalanine (van) | biospider | | Phenylalanine [usan:inn:jan] | biospider | | Phenylalanine, 9CI, USAN; L-form | db_source | | Phenylalanine, l- | biospider | | Phenylalaninum | biospider | | β-phenyl-L-alanine | Generator |
|
|---|
| Chemical Formula | Not Available |
|---|
| IUPAC name | Not Available |
|---|
| InChI Identifier | InChI=1S/C9H11NO2/c10-8(9(11)12)6-7-4-2-1-3-5-7/h1-5,8H,6,10H2,(H,11,12) |
|---|
| InChI Key | COLNVLDHVKWLRT-UHFFFAOYSA-N |
|---|
| Isomeric SMILES | NC(CC1=CC=CC=C1)C(O)=O |
|---|
| Average Molecular Weight | 165.1891 |
|---|
| Monoisotopic Molecular Weight | 165.078978601 |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as phenylalanine and derivatives. Phenylalanine and derivatives are compounds containing phenylalanine or a derivative thereof resulting from reaction of phenylalanine at the amino group or the carboxy group, or from the replacement of any hydrogen of glycine by a heteroatom. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
| Direct Parent | Phenylalanine and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Phenylalanine or derivatives
- 3-phenylpropanoic-acid
- Alpha-amino acid
- Amphetamine or derivatives
- Aralkylamine
- Monocyclic benzene moiety
- Benzenoid
- Amino acid
- Monocarboxylic acid or derivatives
- Carboxylic acid
- Organic nitrogen compound
- Primary amine
- Organic oxide
- Organooxygen compound
- Organonitrogen compound
- Primary aliphatic amine
- Carbonyl group
- Organopnictogen compound
- Organic oxygen compound
- Amine
- Hydrocarbon derivative
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Physico-Chemical Properties - Experimental |
|---|
| Property | Value | Reference |
|---|
| Experimental logP | -1.38 | AVDEEF,A (1997) |
|---|
| Experimental Water Solubility | 26.9 mg/mL at 25 oC | YALKOWSKY,SH & DANNENFELSER,RM (1992) |
|---|
| Melting Point | Mp 283-284° (rapid heat) | DFC |
|---|
|
| Foods of Origin |
|---|
| Food | Content Range | Average | Reference |
|---|
| Food | | | Reference |
|---|
|
| Production Data |
|---|
| Production Method | commercial |
|---|
| Production Method Reference | Not Available |
|---|
| Production Method Reference File | Not Available |
|---|
| Quantity Available | Production upon request, up to 4 g |
|---|
| Delivery Time | Not Available |
|---|
| Storage Form | solid |
|---|
| Storage Conditions | -80°C |
|---|
| Stability | Not Available |
|---|
| Purity | Not Available |
|---|
| Spectra |
|---|
| Spectral Data Upon Request | Not Available |
|---|
| Provider Information |
|---|
|
| Commercial Vendors |
|---|
| AKSci | J93620 |
|---|
| AKSci | K018 |
|---|
| Glentham | GM4385 |
|---|
| Glentham | GM7895 |
|---|
| MetaSci | HMDB0000159 |
|---|
| National Biochemicals Corporation | HMDB0000159 |
|---|
| Toronto Research Chemicals | P319415 |
|---|