| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation date | 2015-10-09 22:27:49 UTC |
|---|
| Update date | 2017-01-19 02:36:11 UTC |
|---|
| FoodComEx ID | PC000062 |
|---|
| FoodDB Record | FDB008293 |
|---|
| Chemical Information |
|---|
| Name | Pyruvic acid |
|---|
| Description | Pyruvic acid is an intermediate compound in the metabolism of carbohydrates, proteins, and fats. In thiamine deficiency, its oxidation is retarded and it accumulates in the tissues, especially in nervous structures (From Stedman, 26th ed.). Biological Source: Intermediate in primary metabolism including fermentation processes. Present in muscle in redox equilibrium with Lactic acid. A common constituent, as a chiral cyclic acetal linked to saccharide residues, of bacterial polysaccharides. Isolated from cane sugar fermentation broth and peppermint. Constituent of Bauhinia purpurea, Cicer arietinum (chickpea), Delonix regia, Pisum sativum (pea) and Trigonella caerulea (sweet trefoil) Use/Importance: Reagent for regeneration of carbonyl compdounds from semicarbazones, phenylhydrazones and oximes. Flavoring ingredient (Dictionary of Organic Compounds); Pyruvate is a key intersection in the network of metabolic pathways. Pyruvate can be converted into carbohydrates via gluconeogenesis, to fatty acids or energy through acetyl-CoA, to the amino acid alanine and to ethanol. Therefore it unites several key metabolic processes.; Pyruvate is an important chemical compound in biochemistry. It is the output of the anaerobic metabolism of glucose known as glycolysis. One molecule of glucose breaks down into two molecules of pyruvate, which are then used to provide further energy, in one of two ways. Pyruvate is converted into acetyl-coenzyme A, which is the main input for a series of reactions known as the Krebs cycle. Pyruvate is also converted to oxaloacetate by an anaplerotic reaction which replenishes Krebs cycle intermediates; alternatively, the oxaloacetate is used for gluconeogenesis. These reactions are named after Hans Adolf Krebs, the biochemist awarded the 1953 Nobel Prize for physiology, jointly with Fritz Lipmann, for research into metabolic processes. The cycle is also called the citric acid cycle, because citric acid is one of the intermediate compounds formed during the reactions.; Pyruvic acid (CH3COCOOH) is an organic acid. It is also a ketone, as well as being the simplest alpha-keto acid. The carboxylate (COOH) ion (anion) of pyruvic acid, CH3COCOO-, is known as pyruvate, and is a key intersection in several metabolic pathways. It can be made from glucose through glycolysis, supplies energy to living cells in the citric acid cycle, and can also be converted to carbohydrates via gluconeogenesis, to fatty acids or energy through acetyl-CoA, to the amino acid alanine and to ethanol.; Pyruvic acid is a colorless liquid with a smell similar to that of acetic acid. It is miscible with water, and soluble in ethanol and diethyl ether. In the laboratory, pyruvic acid may be prepared by heating a mixture of tartaric acid and potassium hydrogen sulfate, by the oxidation of propylene glycol by a strong oxidizer (eg. potassium permanganate or bleach), or by the hydrolysis of acetyl cyanide, formed by reaction of acetyl chloride with potassium cyanide:; Pyruvic acid or pyruvate is a key intermediate in the glycolytic and pyruvate dehydrogenase pathways, which are involved in biological energy production. Pyruvate is widely found in living organisms. It is not an essential nutrient since it can be synthesized in the cells of the body. Certain fruits and vegetables are rich in pyruvate. For example, an average-size red apple contains approximately 450 milligrams. Dark beer and red wine are also rich sources of pyruvate. Recent research suggests that pyruvate in high concentrations may have a role in cardiovascular therapy, as an inotropic agent. Supplements of this dietary substance may also have bariatric and ergogenic applications. Pyruvic acid is isolated from cane sugar fermentation broth, Cicer arietinum (chickpea), Pisum sativum (pea), Trigonella cerulea (sweet trefoil) and peppermint. It can be used as a flavouring ingredient. |
|---|
| CAS Number | 127-17-3 |
|---|
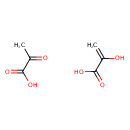
| Structure | |
|---|
| Synonyms | | Synonym | Source |
|---|
| α-ketopropionic acid | biospider | | 2-Ketopropionate | Generator | | 2-ketopropionic acid | biospider | | 2-oxo-propionic acid | biospider | | 2-Oxopropanoate | biospider | | 2-Oxopropanoic acid | biospider | | 2-Oxopropanoic acid, 9CI | db_source | | 2-Oxopropansaeure | ChEBI | | 2-Oxopropionate | biospider | | 2-Oxopropionic acid | biospider | | 2-Oxopropionsaeure | ChEBI | | A-ketopropionate | biospider | | A-ketopropionic acid | biospider | | a-Oxopropionsaeure | Generator | | Acetylformate | biospider | | Acetylformic acid | db_source | | Acide pyruvique | ChEBI | | Alpha-keto propionic acid | biospider | | Alpha-ketopropionate | biospider | | Alpha-ketopropionic acid | biospider | | alpha-Oxopropionsaeure | ChEBI | | Brenztraubensaeure | ChEBI | | BTS | biospider | | CH3COCOOH | biospider | | FEMA 2970 | db_source | | Propanoic acid, 2-oxo- | biospider | | Propanoic acid, 2-oxo- (9CI) | biospider | | Pyroracemate | biospider | | Pyroracemic acid | db_source | | Pyruvate | biospider | | Pyruvic acid (8CI) | biospider | | Sodium 2-oxopropanoate | biospider | | Sodium alpha-ketopropionate | biospider | | α-ketopropionate | Generator | | α-ketopropionic acid | Generator | | α-oxopropionsaeure | Generator |
|
|---|
| Chemical Formula | C6H8O6 |
|---|
| IUPAC name | 2-hydroxyprop-2-enoic acid; 2-oxopropanoic acid |
|---|
| InChI Identifier | InChI=1S/2C3H4O3/c2*1-2(4)3(5)6/h1H3,(H,5,6);4H,1H2,(H,5,6) |
|---|
| InChI Key | UNMYEGNJYFQDES-UHFFFAOYSA-N |
|---|
| Isomeric SMILES | CC(=O)C(O)=O.OC(=C)C(O)=O |
|---|
| Average Molecular Weight | 176.1241 |
|---|
| Monoisotopic Molecular Weight | 176.032087988 |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as alpha-keto acids and derivatives. These are organic compounds containing an aldehyde substituted with a keto group on the adjacent carbon. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Keto acids and derivatives |
|---|
| Sub Class | Alpha-keto acids and derivatives |
|---|
| Direct Parent | Alpha-keto acids and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Alpha-keto acid
- Alpha-hydroxy ketone
- Ketone
- Monocarboxylic acid or derivatives
- Carboxylic acid
- Carboxylic acid derivative
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Carbonyl group
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Not Available |
|---|
| External Descriptors | Not Available |
|---|
| Physico-Chemical Properties - Experimental |
|---|
| Property | Value | Reference |
|---|
| Experimental logP | Not Available | |
|---|
| Experimental Water Solubility | 1000 mg/mL at 20 oC | YALKOWSKY,SH & DANNENFELSER,RM (1992) |
|---|
| Melting Point | Mp ca. 13.6° | DFC |
|---|
|
| Foods of Origin |
|---|
| Food | Content Range | Average | Reference |
|---|
| Food | | | Reference |
|---|
|
| Production Data |
|---|
| Production Method | commercial |
|---|
| Production Method Reference | Not Available |
|---|
| Production Method Reference File | Not Available |
|---|
| Quantity Available | Production upon request, up to 500 mg |
|---|
| Delivery Time | Not Available |
|---|
| Storage Form | liquid |
|---|
| Storage Conditions | -80°C |
|---|
| Stability | Not Available |
|---|
| Purity | Not Available |
|---|
| Spectra |
|---|
| Spectral Data Upon Request | Not Available |
|---|
| Provider Information |
|---|
|
| Commercial Vendors |
|---|
| AKSci | I940 |
|---|
| MetaSci | HMDB0000243 |
|---|
| Sigma-Aldrich | HMDB0000243 |
|---|
| Toronto Research Chemicals | P998895 |
|---|