| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation date | 2015-10-09 22:27:47 UTC |
|---|
| Update date | 2017-01-19 02:36:11 UTC |
|---|
| FoodComEx ID | PC000060 |
|---|
| FoodDB Record | FDB006453 |
|---|
| Chemical Information |
|---|
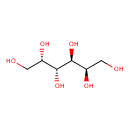
| Name | Galactitol |
|---|
| Description | Galactitol or dulcitol is a sugar alcohol that is a metabolic breakdown product of galactose. Galactitol is an extremely weak basic (essentially neutral) compound (based on its pKa). Galactose is derived from lactose in food (such as dairy products). When lactose is broken down by the enzyme lactase it produces glucose and galactose. It is this galactose that is broken down to galactitol via a reaction catalyzed by aldose reductase. Galactitol has a slightly sweet taste. When present in sufficiently high levels, galactitol can act as a metabotoxin, a neurotoxin, and a hepatotoxin. A neurotoxin is a compound that disrupts or attacks neural cells and neural tissue. A hepatotoxin as a compound that disrupts or attacks liver tissue or liver cells. A metabotoxin is an endogenously produced metabolite that causes adverse health effects at chronically high levels. Chronically high levels of galactitol are associated with at least two inborn errors of metabolism, including galactosemia and galactosemia type II. Galactosemia is a rare genetic metabolic disorder that affects an individual's ability to metabolize the sugar galactose properly. Excess lactose consumption in individuals with galactose intolerance or galactosemia activates aldose reductase to produce galactitol, thus depleting NADPH and leading to lowered glutathione reductase activity. As a result, hydrogen peroxide or other free radicals accumulate causing serious oxidative damage to various cells and tissues. In individuals with galactosemia, the enzymes needed for the further metabolism of galactose (galactose-1-phosphate uridyltransferase) are severely diminished or missing entirely, leading to toxic levels of galactose 1-phosphate, galactitol, and galactonate. High levels of galactitol in infants are specifically associated with hepatomegaly (an enlarged liver), cirrhosis, renal failure, cataracts, vomiting, seizure, hypoglycemia, lethargy, brain damage, and ovarian failure. Outside of the human body, Galactitol has been detected, but not quantified in, several different foods, such as common buckwheats, winter squash, calabash, black walnuts, and peanuts. This could make galactitol a potential biomarker for the consumption of these foods. |
|---|
| CAS Number | 608-66-2 |
|---|
| Structure | |
|---|
| Synonyms | | Synonym | Source |
|---|
| (2R,3S,4R,5S)-Hexane-1,2,3,4,5,6-hexol | ChEBI | | Ambap5938 | HMDB | | D-Dulcitol | ChEBI | | D-Galactitol | ChEBI | | Dulcite | db_source | | Dulcitol | db_source | | Dulcose | db_source | | Euonymit | db_source | | galacto-Hexitol | db_source | | Hexitol | HMDB | | L-Galactitol | ChEBI | | Melampyrin | db_source | | Melampyrit | ChEBI | | Melampyrite | HMDB | | Melampyrum | db_source | | Meso-galactitol | HMDB |
|
|---|
| Chemical Formula | C6H14O6 |
|---|
| IUPAC name | (2R,3S,4R,5S)-hexane-1,2,3,4,5,6-hexol |
|---|
| InChI Identifier | InChI=1S/C6H14O6/c7-1-3(9)5(11)6(12)4(10)2-8/h3-12H,1-2H2/t3-,4+,5+,6- |
|---|
| InChI Key | FBPFZTCFMRRESA-GUCUJZIJSA-N |
|---|
| Isomeric SMILES | OC[C@H](O)[C@@H](O)[C@@H](O)[C@H](O)CO |
|---|
| Average Molecular Weight | 182.1718 |
|---|
| Monoisotopic Molecular Weight | 182.07903818 |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as sugar alcohols. These are hydrogenated forms of carbohydrate in which the carbonyl group (aldehyde or ketone, reducing sugar) has been reduced to a primary or secondary hydroxyl group. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic oxygen compounds |
|---|
| Class | Organooxygen compounds |
|---|
| Sub Class | Carbohydrates and carbohydrate conjugates |
|---|
| Direct Parent | Sugar alcohols |
|---|
| Alternative Parents | |
|---|
| Substituents | - Sugar alcohol
- Monosaccharide
- Secondary alcohol
- Polyol
- Hydrocarbon derivative
- Primary alcohol
- Alcohol
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Physico-Chemical Properties - Experimental |
|---|
| Property | Value | Reference |
|---|
| Experimental logP | -3.10 | HANSCH,C ET AL. (1995) |
|---|
| Experimental Water Solubility | 31 mg/mL at 15 oC | YALKOWSKY,SH & DANNENFELSER,RM (1992) |
|---|
| Melting Point | 189.5 oC | |
|---|
|
| Foods of Origin |
|---|
| Food | Content Range | Average | Reference |
|---|
| Food | | | Reference |
|---|
|
| Production Data |
|---|
| Production Method | commercial |
|---|
| Production Method Reference | Not Available |
|---|
| Production Method Reference File | Not Available |
|---|
| Quantity Available | Production upon request, up to 2 g |
|---|
| Delivery Time | Not Available |
|---|
| Storage Form | solid |
|---|
| Storage Conditions | -80°C |
|---|
| Stability | Not Available |
|---|
| Purity | Not Available |
|---|
| Spectra |
|---|
| Spectral Data Upon Request | Not Available |
|---|
| Provider Information |
|---|
|
| Commercial Vendors |
|---|
| AKSci | C895 |
|---|
| Glentham | GC5527 |
|---|
| Human Metabolome Library | HMDB0000107 |
|---|
| MetaSci | HMDB0000107 |
|---|
| Toronto Research Chemicals | D720500 |
|---|