| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation date | 2015-10-09 22:27:47 UTC |
|---|
| Update date | 2017-01-19 02:36:11 UTC |
|---|
| FoodComEx ID | PC000059 |
|---|
| FoodDB Record | FDB022188 |
|---|
| Chemical Information |
|---|
| Name | 1-Methylnicotinamide |
|---|
| Description | 1-Methylnicotinamide is a metabolite of nicotinamide and is produced primarily in the liver. It has anti-inflammatory properties (PMID 16197374). It is a product of nicotinamide N-methyltransferase [EC 2.1.1.1] in the pathway of nicotinate and nicotinamide metabolism (KEGG). 1-Methylnicotinamide may be an endogenous activator of prostacyclin production and thus may regulate thrombotic as well as inflammatory processes in the cardiovascular system (PMID: 17641676). [HMDB] |
|---|
| CAS Number | 3106-60-3 |
|---|
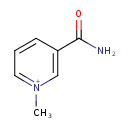
| Structure | |
|---|
| Synonyms | | Synonym | Source |
|---|
| 1-Methyl-3-carbamoylpyridinium | hmdb | | 1-Methyl-3-carbamoylpyridinium cation | hmdb | | 1-Methylnicotinamide | hmdb | | 1-Methylnicotinamide cation | ChEBI | | 3-Amido-N-methylpyridinium: 1-methyl-3-Pyridinecarboxamide | hmdb | | 3-carbamoyl-1-methyl-Pyridinium | hmdb | | I-methyl nicotinamide | hmdb | | N-1-methylnicotinamide | hmdb | | N-Methyl-3-carbamidopyridinium | hmdb | | N-Methyl-3-carbamoylpyridinium ion | hmdb | | N'-methylnicotinamide | hmdb | | N'methylnicotinamide | hmdb | | N1-Methylnicotinamide | hmdb | | Trigonellinamide | hmdb |
|
|---|
| Chemical Formula | C7H9N2O |
|---|
| IUPAC name | 3-carbamoyl-1-methylpyridin-1-ium |
|---|
| InChI Identifier | InChI=1S/C7H8N2O/c1-9-4-2-3-6(5-9)7(8)10/h2-5H,1H3,(H-,8,10)/p+1 |
|---|
| InChI Key | LDHMAVIPBRSVRG-UHFFFAOYSA-O |
|---|
| Isomeric SMILES | C[N+]1=CC=CC(=C1)C(N)=O |
|---|
| Average Molecular Weight | 137.1592 |
|---|
| Monoisotopic Molecular Weight | 137.07148792 |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as nicotinamides. These are heterocyclic aromatic compounds containing a pyridine ring substituted at position 3 by a carboxamide group. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Pyridines and derivatives |
|---|
| Sub Class | Pyridinecarboxylic acids and derivatives |
|---|
| Direct Parent | Nicotinamides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Nicotinamide
- N-methylpyridinium
- Pyridinium
- Heteroaromatic compound
- Vinylogous amide
- Carboxamide group
- Primary carboxylic acid amide
- Carboxylic acid derivative
- Azacycle
- Organic nitrogen compound
- Organic oxygen compound
- Hydrocarbon derivative
- Organic oxide
- Organooxygen compound
- Organonitrogen compound
- Organopnictogen compound
- Organic cation
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Physico-Chemical Properties - Experimental |
|---|
| Property | Value | Reference |
|---|
| Experimental logP | Not Available | |
|---|
| Experimental Water Solubility | Not Available | |
|---|
| Melting Point | Not Available | |
|---|
|
| Foods of Origin |
|---|
| Food | Content Range | Average | Reference |
|---|
| Food | | | Reference |
|---|
|
| Production Data |
|---|
| Production Method | commercial |
|---|
| Production Method Reference | Not Available |
|---|
| Production Method Reference File | Not Available |
|---|
| Quantity Available | Production upon request, up to 30 mg |
|---|
| Delivery Time | Not Available |
|---|
| Storage Form | solid |
|---|
| Storage Conditions | -80°C |
|---|
| Stability | Not Available |
|---|
| Purity | Not Available |
|---|
| Spectra |
|---|
| Spectral Data Upon Request | Not Available |
|---|
| Provider Information |
|---|
|
| Commercial Vendors |
|---|
| Not Available |