| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation date | 2015-10-09 22:27:46 UTC |
|---|
| Update date | 2017-01-19 02:36:11 UTC |
|---|
| FoodComEx ID | PC000056 |
|---|
| FoodDB Record | FDB012680 |
|---|
| Chemical Information |
|---|
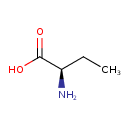
| Name | D-2-Aminobutanoic acid |
|---|
| Description | Constituent of seedlings of Glycine max (soybean), Dolichos lablab (hyacinth bean), Canavalia gladiata (swordbean), Arachis hypogaea (peanut), Pisum sativum (pea), Phaseolus vulgaris (kidney bean) and Vigna sesquipedalis (asparagus bean) after hydrolysis
alpha-Aminobutyric acid (AABA) is an isomer of the amino acid aminobutyric acid. It is a key intermediate in the biosynthesis of ophthalmic acid or ophthalmate. D-2-Aminobutanoic acid is found in many foods, some of which are nuts, common pea, yellow wax bean, and pulses. |
|---|
| CAS Number | 2623-91-8 |
|---|
| Structure | |
|---|
| Synonyms | | Synonym | Source |
|---|
| (2R)-2-Aminobutanoate | biospider | | (2R)-2-Aminobutanoic acid | biospider | | (2R)-2-Aminobutyrate | Generator | | (2R)-2-Aminobutyric acid | ChEBI | | (R)-(-)-2-Aminobutyric acid | biospider | | (R)-2-amino-Butanoate | HMDB | | (R)-2-amino-Butanoic acid | HMDB | | (R)-2-Aminobutanoate | biospider | | (R)-2-Aminobutanoic acid | biospider | | (R)-2-Aminobutyrate | Generator | | (R)-2-Aminobutyric acid | ChEBI | | 2-Aminobutanoic acid, 9CI; D-form | db_source | | a-Aminobutyrate | Generator | | a-Aminobutyric acid | Generator | | alpha-Aminobutyrate | Generator | | D-(-)-2-Aminobutyrate | Generator | | D-(-)-2-Aminobutyric acid | biospider | | D-2-Aminobutanoate | biospider | | D-2-Aminobutanoic acid | biospider | | D-2-Aminobuttersaeure | ChEBI | | D-2-Aminobutyrate | biospider | | D-2-Aminobutyric acid | biospider | | D-a-Aminobutyric acid | HMDB | | D-AABA | manual | | D-alpha-aminobutyric acid | manual | | delta-(-)-2-Aminobutyric acid | HMDB | | delta-2-Aminobutanoate | HMDB | | delta-2-Aminobutanoic acid | HMDB | | delta-2-Aminobuttersaeure | HMDB | | delta-2-Aminobutyrate | HMDB | | delta-2-Aminobutyric acid | HMDB | | delta-alpha-Aminobutyric acid | HMDB | | α-aminobutyrate | Generator | | α-aminobutyric acid | Generator |
|
|---|
| Chemical Formula | C4H9NO2 |
|---|
| IUPAC name | (2R)-2-aminobutanoic acid |
|---|
| InChI Identifier | InChI=1S/C4H9NO2/c1-2-3(5)4(6)7/h3H,2,5H2,1H3,(H,6,7)/t3-/m1/s1 |
|---|
| InChI Key | QWCKQJZIFLGMSD-GSVOUGTGSA-N |
|---|
| Isomeric SMILES | CC[C@@H](N)C(O)=O |
|---|
| Average Molecular Weight | 103.1198 |
|---|
| Monoisotopic Molecular Weight | 103.063328537 |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as d-alpha-amino acids. These are alpha amino acids which have the D-configuration of the alpha-carbon atom. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
| Direct Parent | D-alpha-amino acids |
|---|
| Alternative Parents | |
|---|
| Substituents | - D-alpha-amino acid
- Fatty acid
- Amino acid
- Carboxylic acid
- Monocarboxylic acid or derivatives
- Amine
- Hydrocarbon derivative
- Organic oxide
- Organopnictogen compound
- Primary amine
- Organooxygen compound
- Organonitrogen compound
- Organic oxygen compound
- Primary aliphatic amine
- Organic nitrogen compound
- Carbonyl group
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Physico-Chemical Properties - Experimental |
|---|
| Property | Value | Reference |
|---|
| Experimental logP | Not Available | |
|---|
| Experimental Water Solubility | Not Available | |
|---|
| Melting Point | Mp 292° dec. | DFC |
|---|
|
| Foods of Origin |
|---|
| Food | Content Range | Average | Reference |
|---|
| Food | | | Reference |
|---|
|
| Production Data |
|---|
| Production Method | commercial |
|---|
| Production Method Reference | Not Available |
|---|
| Production Method Reference File | Not Available |
|---|
| Quantity Available | Production upon request, up to 4 g |
|---|
| Delivery Time | Not Available |
|---|
| Storage Form | solid |
|---|
| Storage Conditions | -80°C |
|---|
| Stability | Not Available |
|---|
| Purity | Not Available |
|---|
| Spectra |
|---|
| Spectral Data Upon Request | Not Available |
|---|
| Provider Information |
|---|
|
| Commercial Vendors |
|---|
| AKSci | E550 |
|---|
| MetaSci | HMDB0000650 |
|---|
| Sigma-Aldrich | HMDB0000650 |
|---|
| Toronto Research Chemicals | A602915 |
|---|