| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation date | 2015-10-09 22:27:42 UTC |
|---|
| Update date | 2017-01-19 02:36:11 UTC |
|---|
| FoodComEx ID | PC000047 |
|---|
| FoodDB Record | FDB012810 |
|---|
| Chemical Information |
|---|
| Name | Cholic acid |
|---|
| Description | Emulsifying agent in foods
A major primary bile acid produced in the liver and usually conjugated with glycine or taurine. It facilitates fat absorption and cholesterol excretion. ; Bile acids are steroid acids found predominantly in bile of mammals. The distinction between different bile acids is minute, depends only on presence or absence of hydroxyl groups on positions 3, 7, and 12. ; Bile acids are physiological detergents that facilitate excretion, absorption, and transport of fats and sterols in the intestine and liver. Bile acids are also steroidal amphipathic molecules derived from the catabolism of cholesterol. They modulate bile flow and lipid secretion, are essential for the absorption of dietary fats and vitamins, and have been implicated in the regulation of all the key enzymes involved in cholesterol homeostasis. ; Bile acids recirculate through the liver, bile ducts, small intestine and portal vein to form an enterohepatic circuit. They exist as anions at physiological pH and, consequently, require a carrier for transport across the membranes of the enterohepatic tissues. The unique detergent properties of bile acids are essential for the digestion and intestinal absorption of hydrophobic nutrients. Bile acids have potent toxic properties (e.g., membrane disruption) and there are a plethora of mechanisms to limit their accumulation in blood and tissues. (PMID: 11316487, 16037564, 12576301, 11907135); Cholic acid is a bile acid, a white crystalline substance insoluble in water (soluble in alcohol and acetic acid), with a melting point of 200-201 °C. Salts of cholic acid are called cholates. Cholic acid, along with chenodeoxycholic acid, is one of two major bile acids produced by the liver where it is synthesized from cholesterol. Of the two major bile acids, cholate derivatives represent approximately eighty percent of all bile acids. These derivatives are made from cholyl-CoA which forms a conjugate with either glycine, or taurine, yielding glycocholic and taurocholic acid respectively. |
|---|
| CAS Number | 81-25-4 |
|---|
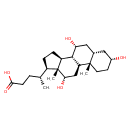
| Structure | |
|---|
| Synonyms | | Synonym | Source |
|---|
| (3a,5b,7a,12a)-3,7,12-Trihydroxycholan-24-Oate | Generator | | (3a,5b,7a,12a)-3,7,12-Trihydroxycholan-24-Oic acid | Generator | | (3alpha,5beta,7alpha,12alpha)-3,7,12-Trihydroxycholan-24-Oate | Generator | | (3alpha,5beta,7alpha,12alpha)-3,7,12-Trihydroxycholan-24-Oic acid | ChEBI | | (3α,5β,7α,12α)-3,7,12-trihydroxycholan-24-Oate | Generator | | (3α,5β,7α,12α)-3,7,12-trihydroxycholan-24-Oic acid | Generator | | 17b-[1-Methyl-3-carboxypropyl]etiocholane-3a,7a,12a-triol | biospider | | 3-&alpha,7-&alpha,12-&alpha-trihydroxy-5-&beta-cholanate | biospider | | 3,7,12-Trihydroxy-cholan-24-oic acid | biospider | | 3,7,12-Trihydroxycholanic acid | biospider | | 3a,7a,12a-Trihydroxy-5b-cholan-24-oate | biospider | | 3a,7a,12a-Trihydroxy-5b-cholan-24-oic acid | biospider | | 3a,7a,12a-Trihydroxy-5b-cholanate | biospider | | 3a,7a,12a-Trihydroxy-5b-cholanic acid | biospider | | 3a,7a,12a-Trihydroxy-5b-cholanoate | biospider | | 3a,7a,12a-Trihydroxy-5b-cholanoic acid | biospider | | 3a,7a,12a-Trihydroxy-b-cholanate | biospider | | 3a,7a,12a-Trihydroxy-b-cholanic acid | biospider | | 3a,7a,12a-Trihydroxy-beta-cholanate | biospider | | 3a,7a,12a-Trihydroxy-beta-cholanic acid | biospider | | 3a,7a,12a-Trihydroxycholanate | biospider | | 3a,7a,12a-Trihydroxycholanic acid | biospider | | 3alpha,7alpha-Dihydroxy-5beta-cholanic acid | biospider | | 3alpha,7alpha,12alpha-trihydroxy-5beta-cholan-24-oic acid | biospider | | 3alpha,7alpha,12alpha-Trihydroxy-5beta-cholanate | biospider | | 3alpha,7alpha,12alpha-trihydroxy-5beta-cholanic acid | biospider | | 3alpha,7alpha,12alpha-Trihydroxy-5beta-cholanoic acid | biospider | | 3alpha,7alpha,12alpha-Trihydroxy-beta-cholanic acid | biospider | | 3alpha,7alpha,12alpha-Trihydroxycholanic acid | biospider | | 3α,7α,12α-trihydroxy-5β-cholanate | Generator | | 3α,7α,12α-trihydroxy-5β-cholanic acid | Generator | | 5b-Cholanic acid-3a,7a,12a-triol | biospider | | 5b-Cholate | biospider | | 5b-Cholic acid | biospider | | 5beta-Cholan-24-oic acid, 3alpha,7alpha,12alpha-trihydroxy- | biospider | | 5beta-Cholanic acid-3alpha,7alpha-diol | biospider | | Chenodiol | biospider | | Cholagit | db_source | | Cholalate | biospider | | Cholalic acid | db_source | | Cholalin | biospider | | Cholan-24-oic acid | biospider | | Cholanic acid | biospider | | Cholate | biospider | | Cholic acid | db_source | | Cholic acid, 5beta- | biospider | | Cholsaeure | ChEBI | | Colalin | db_source | | Hypocholate | db_source | | NSC 6135 | db_source |
|
|---|
| Chemical Formula | C24H40O5 |
|---|
| IUPAC name | (4R)-4-[(1S,2S,5R,7S,9R,10R,11S,14R,15R,16S)-5,9,16-trihydroxy-2,15-dimethyltetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadecan-14-yl]pentanoic acid |
|---|
| InChI Identifier | InChI=1S/C24H40O5/c1-13(4-7-21(28)29)16-5-6-17-22-18(12-20(27)24(16,17)3)23(2)9-8-15(25)10-14(23)11-19(22)26/h13-20,22,25-27H,4-12H2,1-3H3,(H,28,29) |
|---|
| InChI Key | BHQCQFFYRZLCQQ-UHFFFAOYSA-N |
|---|
| Isomeric SMILES | CC(CCC(O)=O)C1CCC2C3C(O)CC4CC(O)CCC4(C)C3CC(O)C12C |
|---|
| Average Molecular Weight | 408.5714 |
|---|
| Monoisotopic Molecular Weight | 408.28757439 |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as trihydroxy bile acids, alcohols and derivatives. These are prenol lipids structurally characterized by a bile acid or alcohol which bears three hydroxyl groups. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Steroids and steroid derivatives |
|---|
| Sub Class | Bile acids, alcohols and derivatives |
|---|
| Direct Parent | Trihydroxy bile acids, alcohols and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Trihydroxy bile acid, alcohol, or derivatives
- 3-hydroxysteroid
- 12-hydroxysteroid
- 7-hydroxysteroid
- 3-alpha-hydroxysteroid
- Hydroxysteroid
- Cyclic alcohol
- Secondary alcohol
- Carboxylic acid derivative
- Carboxylic acid
- Polyol
- Monocarboxylic acid or derivatives
- Organic oxide
- Alcohol
- Organic oxygen compound
- Hydrocarbon derivative
- Carbonyl group
- Organooxygen compound
- Aliphatic homopolycyclic compound
|
|---|
| Molecular Framework | Aliphatic homopolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Physico-Chemical Properties - Experimental |
|---|
| Property | Value | Reference |
|---|
| Experimental logP | 2.02 | RODA,A ET AL. (1990) |
|---|
| Experimental Water Solubility | 0.175 mg/mL at 20 oC | YALKOWSKY,SH & DANNENFELSER,RM (1992) |
|---|
| Melting Point | Mp 197° (anhyd.) | DFC |
|---|
|
| Foods of Origin |
|---|
| Food | Content Range | Average | Reference |
|---|
| Food | | | Reference |
|---|
|
| Production Data |
|---|
| Production Method | commercial |
|---|
| Production Method Reference | Not Available |
|---|
| Production Method Reference File | Not Available |
|---|
| Quantity Available | Production upon request, up to 3 g |
|---|
| Delivery Time | Not Available |
|---|
| Storage Form | solid |
|---|
| Storage Conditions | -80°C |
|---|
| Stability | Not Available |
|---|
| Purity | Not Available |
|---|
| Spectra |
|---|
| Spectral Data Upon Request | Not Available |
|---|
| Provider Information |
|---|
|
| Commercial Vendors |
|---|
| AKSci | K446 |
|---|
| Cayman Chemical | 20250 |
|---|
| Eastman Chemical Company | HMDB0000619 |
|---|
| Glentham | GD0884 |
|---|
| MetaSci | HMDB0000619 |
|---|
| Toronto Research Chemicals | C432600 |
|---|