| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation date | 2015-10-09 22:27:38 UTC |
|---|
| Update date | 2017-01-19 02:36:11 UTC |
|---|
| FoodComEx ID | PC000039 |
|---|
| FoodDB Record | FDB022283 |
|---|
| Chemical Information |
|---|
| Name | Pimelic acid |
|---|
| Description | A group of compounds that are derivatives of heptanedioic acid with the general formula R-C7H11O4.
Pimelic acid is the organic compound with the formula HO2C(CH2)5CO2H. Derivatives of pimelic acid are involved in the biosynthesis of the amino acid called lysine. Pimelic acid is one methylene longer than a related dicarboxylic acid, adipic acid, a precursor to many polyesters and polyamides.
Pimelic acid has been synthesized from cyclohexanone and from salicylic acid.[1] In the former route, the additional carbon is suppled by dimethyloxalate, which reacts with the enolate. [HMDB] |
|---|
| CAS Number | 111-16-0 |
|---|
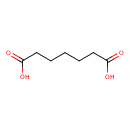
| Structure | |
|---|
| Synonyms | | Synonym | Source |
|---|
| 1,5-Pentanedicarboxylate | Generator | | 1,5-Pentanedicarboxylic acid | ChEBI | | 1,7-Heptanedioate | hmdb | | 1,7-Heptanedioic acid | hmdb | | 6-carboxyhexanoate | hmdb | | 6-carboxyhexanoic acid | hmdb | | Heptandioate | hmdb | | Heptandioic acid | hmdb | | Heptane-1,7-dioate | hmdb | | Heptane-1,7-dioic acid | hmdb | | Heptanedioate | hmdb | | Heptanedioic acid | hmdb | | Pilerate | hmdb | | Pileric acid | hmdb | | Pimelate | hmdb | | Pimelic acid | hmdb |
|
|---|
| Chemical Formula | C7H12O4 |
|---|
| IUPAC name | heptanedioic acid |
|---|
| InChI Identifier | InChI=1S/C7H12O4/c8-6(9)4-2-1-3-5-7(10)11/h1-5H2,(H,8,9)(H,10,11) |
|---|
| InChI Key | WLJVNTCWHIRURA-UHFFFAOYSA-N |
|---|
| Isomeric SMILES | OC(=O)CCCCCC(O)=O |
|---|
| Average Molecular Weight | 160.1678 |
|---|
| Monoisotopic Molecular Weight | 160.073558872 |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as medium-chain fatty acids. These are fatty acids with an aliphatic tail that contains between 4 and 12 carbon atoms. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Fatty Acyls |
|---|
| Sub Class | Fatty acids and conjugates |
|---|
| Direct Parent | Medium-chain fatty acids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Medium-chain fatty acid
- Dicarboxylic acid or derivatives
- Carboxylic acid
- Carboxylic acid derivative
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Carbonyl group
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Physico-Chemical Properties - Experimental |
|---|
| Property | Value | Reference |
|---|
| Experimental logP | Not Available | |
|---|
| Experimental Water Solubility | Not Available | |
|---|
| Melting Point | Not Available | |
|---|
|
| Foods of Origin |
|---|
| Food | Content Range | Average | Reference |
|---|
| Food | | | Reference |
|---|
|
| Production Data |
|---|
| Production Method | commercial |
|---|
| Production Method Reference | Not Available |
|---|
| Production Method Reference File | Not Available |
|---|
| Quantity Available | Production upon request, up to 4 g |
|---|
| Delivery Time | Not Available |
|---|
| Storage Form | solid |
|---|
| Storage Conditions | -80°C |
|---|
| Stability | Not Available |
|---|
| Purity | Not Available |
|---|
| Spectra |
|---|
| Spectral Data Upon Request | Not Available |
|---|
| Provider Information |
|---|
|
| Commercial Vendors |
|---|
| AKSci | H404 |
|---|
| AKSci | J92528 |
|---|
| MetaSci | HMDB0000857 |
|---|
| Sigma-Aldrich | HMDB0000857 |
|---|
| Toronto Research Chemicals | P445040 |
|---|