4-Hydroxyphenylpyruvic acid (PC000034)
| Record Information | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Version | 1.0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Creation date | 2015-10-09 22:27:36 UTC | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Update date | 2017-01-19 02:36:10 UTC | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| FoodComEx ID | PC000034 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| FoodDB Record | FDB022193 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Information | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Name | 4-Hydroxyphenylpyruvic acid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description | 4-Hydroxyphenylpyruvic acid (4-HPPA) is a keto acid. It is a product of the enzyme (R)-4-hydroxyphenyllactate dehydrogenase [EC 1.1.1.222] and is formed during tyrosine metabolism (KEGG). There are two isomers of HPPA, specifically 4HPPA and 3HPPA, of which 4HPPA is the most common. The enzyme 4-hydroxyphenylpyruvic acid dioxygenase (HPD) catalyzes the reaction of 4-hydroxyphenylpyruvic acid to homogentisic acid in the tyrosine catabolism pathway. A deficiency in the catalytic activity of HPD is known to lead to tyrosinemia type III, an autosomal recessive disorder characterized by elevated levels of blood tyrosine and massive excretion of tyrosine derivatives into urine. It has been shown that hawkinsinuria, an autosomal dominant disorder characterized by the excretion of 'hawkinsin,' may also be a result of HPD deficiency (PMID: 11073718). [HMDB] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS Number | 156-39-8 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
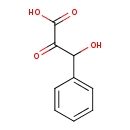
| Structure | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synonyms |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Formula | C9H8O4 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IUPAC name | 3-hydroxy-2-oxo-3-phenylpropanoic acid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Identifier | InChI=1S/C9H8O4/c10-7(8(11)9(12)13)6-4-2-1-3-5-6/h1-5,7,10H,(H,12,13) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Key | ZHLWCBHWYUISFY-UHFFFAOYSA-N | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Isomeric SMILES | OC(C(=O)C(O)=O)C1=CC=CC=C1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Average Molecular Weight | 180.1574 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Monoisotopic Molecular Weight | 180.042258744 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Taxonomy | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description | Belongs to the class of organic compounds known as phenylpyruvic acid derivatives. Phenylpyruvic acid derivatives are compounds containing a phenylpyruvic acid moiety, which consists of a phenyl group substituted at the second position by an pyruvic acid. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Kingdom | Organic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Super Class | Benzenoids | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Benzene and substituted derivatives | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sub Class | Phenylpyruvic acid derivatives | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Direct Parent | Phenylpyruvic acid derivatives | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alternative Parents | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Substituents |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Framework | Aromatic homomonocyclic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Descriptors | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physico-Chemical Properties - Experimental | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Foods of Origin | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Production Data | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Production Method | commercial | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Production Method Reference | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Production Method Reference File | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Quantity Available | Production upon request, up to 30 mg | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Delivery Time | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Storage Form | solid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Storage Conditions | -80°C | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Stability | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Purity | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectral Data Upon Request | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Provider Information | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Commercial Vendors | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| AKSci | C107 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| MetaSci | HMDB0000707 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sigma-Aldrich | HMDB0000707 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Toronto Research Chemicals | H949870 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||