| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation date | 2015-10-09 22:27:35 UTC |
|---|
| Update date | 2017-01-19 02:36:10 UTC |
|---|
| FoodComEx ID | PC000033 |
|---|
| FoodDB Record | FDB010547 |
|---|
| Chemical Information |
|---|
| Name | Myo-Inositol |
|---|
| Description | Myo-Inositol is an inositol isoform. Inositol is a derivative of cyclohexane with six hydroxyl groups, making it a polyol. It also is known as a sugar alcohol, having the same molecular formula as glucose or other hexoses. Inositol exists in nine possible stereoisomers, including scyllo-inositol, myo-inositol (the most abundant), muco-inositol, D-chiro-inositol, L-chiro-inositol, neo-inositol, allo-inositol, epi-inositol, and cis-inositol. In humans, most inositol is synthesized in the kidneys, typically in amounts of a few grams per day. It is found in many foods, particularly in cereals with high bran content. It is an isomer of glucose that has traditionally been a B vitamin although it has an uncertain status as a vitamin and a deficiency syndrome has not been identified in humans. Inositol is a cyclic polyalcohol that plays an important role as a second messenger in a cell, in the form of inositol phosphates. Inositol phospholipids are important in signal transduction. A possible health effect of inositols, including myo-inositol, was recently reviewed. The review concluded that myo-inositol, together with chiro-inositol, at a 40:1 ratio, were an important therapeutic strategy for the improvement of metabolic, hormonal, and reproductive aspects of polycystic ovarian syndrome (PMID: 29309199). |
|---|
| CAS Number | 87-89-8 |
|---|
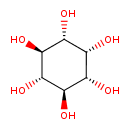
| Structure | |
|---|
| Synonyms | | Synonym | Source |
|---|
| (1a,2a,3a,4b,5a,6b)-Cyclohexanehexol | db_source | | (1R,2R,3S,4S,5R,6S)-Cyclohexane-1,2,3,4,5,6-hexol | ChEBI | | 1,2,3,4,5,6-HEXAHYDROXY-cyclohexane | ChEBI | | 1,2,3,5/4,6-cyclohexanehexol | ChEBI | | 1,2,3,5/4,6-Inositol | db_source | | 1D-myo-Inositol | ChEBI | | 1L-myo-Inositol | ChEBI | | Bios I | ChEBI | | cis-1,2,3,5-trans-4,6-Cyclohexanehexol | ChEBI | | Cyclohexitol | ChEBI | | D-myo-Inositol | ChEBI | | Dambose | ChEBI | | i-Inositol | db_source | | Inosite | ChEBI | | Inositol | ChEBI | | Ins | ChEBI | | Iso-inositol | HMDB | | L-myo-Inositol | ChEBI | | Meat sugar | ChEBI | | meso-Inositol | db_source | | MI | HMDB | | Myoinosite | HMDB | | Myoinositol | ChEBI | | Nucitol | db_source | | Phaseomannite | HMDB | | Rat antispectacled eye factor | HMDB |
|
|---|
| Chemical Formula | C6H12O6 |
|---|
| IUPAC name | (1R,2R,3r,4S,5S,6s)-cyclohexane-1,2,3,4,5,6-hexol |
|---|
| InChI Identifier | InChI=1S/C6H12O6/c7-1-2(8)4(10)6(12)5(11)3(1)9/h1-12H/t1-,2-,3-,4+,5-,6- |
|---|
| InChI Key | CDAISMWEOUEBRE-GPIVLXJGSA-N |
|---|
| Isomeric SMILES | O[C@H]1[C@H](O)[C@@H](O)[C@H](O)[C@H](O)[C@@H]1O |
|---|
| Average Molecular Weight | 180.1559 |
|---|
| Monoisotopic Molecular Weight | 180.063388116 |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as cyclohexanols. Cyclohexanols are compounds containing an alcohol group attached to a cyclohexane ring. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic oxygen compounds |
|---|
| Class | Organooxygen compounds |
|---|
| Sub Class | Alcohols and polyols |
|---|
| Direct Parent | Cyclohexanols |
|---|
| Alternative Parents | |
|---|
| Substituents | - Cyclohexanol
- Sugar alcohol
- Cyclitol or derivatives
- Cyclic alcohol
- Polyol
- Hydrocarbon derivative
- Aliphatic homomonocyclic compound
|
|---|
| Molecular Framework | Aliphatic homomonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Physico-Chemical Properties - Experimental |
|---|
| Property | Value | Reference |
|---|
| Experimental logP | Not Available | |
|---|
| Experimental Water Solubility | 143 mg/mL at 19 oC | YALKOWSKY,SH & DANNENFELSER,RM (1992) |
|---|
| Melting Point | Mp 225° | DFC |
|---|
|
| Foods of Origin |
|---|
| Food | Content Range | Average | Reference |
|---|
| Food | | | Reference |
|---|
|
| Production Data |
|---|
| Production Method | commercial |
|---|
| Production Method Reference | Not Available |
|---|
| Production Method Reference File | Not Available |
|---|
| Quantity Available | Production upon request, up to 2 g |
|---|
| Delivery Time | Not Available |
|---|
| Storage Form | solid |
|---|
| Storage Conditions | -80°C |
|---|
| Stability | Not Available |
|---|
| Purity | Not Available |
|---|
| Spectra |
|---|
| Spectral Data Upon Request | Not Available |
|---|
| Provider Information |
|---|
|
| Commercial Vendors |
|---|
| AKSci | K572 |
|---|
| Glentham | GC4902 |
|---|
| MetaSci | HMDB0000211 |
|---|
| Sigma-Aldrich | HMDB0000211 |
|---|
| Toronto Research Chemicals | I665995 |
|---|