| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation date | 2015-10-09 22:33:28 UTC |
|---|
| Update date | 2017-01-19 02:36:42 UTC |
|---|
| FoodComEx ID | PC000936 |
|---|
| FoodDB Record | FDB022939 |
|---|
| Chemical Information |
|---|
| Name | Methylcobalamin |
|---|
| Description | The name vitamin B12 is used in two different ways. In a broad sense it refers to a group of cobalt-containing compounds known as cobalamins - cyanocobalamin (an artifact formed as a result of the use of cyanide in the purification procedures), hydroxocobalamin and the two coenzyme forms of B12, methylcobalamin (MeB12) and 5-deoxyadenosylcobalamin (adenosylcobalamin - AdoB12). In a more specific way, the term B12 is used to refer to only one of these forms, cyanocobalamin, which is the principal B12 form used for foods and in nutritional supplements.
B12 cannot be made by plants or by animals, as the only type of organisms that have the enzymes required for the synthesis of B12 are bacteria and archaea. The total synthesis of B12 was reported in 1973 by Robert Burns Woodward, and remains one of the classic feats of total synthesis.
Cyanocobalamin is a vitamin commonly known as vitamin B12 (or B12 for short). [HMDB] |
|---|
| CAS Number | 13422-55-4 |
|---|
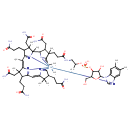
| Structure | |
|---|
| Synonyms | | Synonym | Source |
|---|
| Algobaz | hmdb | | Co-Methylcobalamin | hmdb | | Hitocobamin M | hmdb | | MeCbl | hmdb | | Mecobalamin | hmdb | | Methycobal | hmdb | | Methyl cobalamine | hmdb | | Methyl vitamin B12 | hmdb | | Methyl-5,6-dimethylbenzimidazolylcobalamin | hmdb | | Methyl-B12 | hmdb | | Methylcob(III)alamin | hmdb | | Methylcobalamin | hmdb | | Methylcobaz | hmdb |
|
|---|
| Chemical Formula | C63H91CoN13O14P |
|---|
| IUPAC name | Not Available |
|---|
| InChI Identifier | InChI=1S/C62H89N13O14P.CH3.Co/c1-29-20-39-40(21-30(29)2)75(28-70-39)57-52(84)53(41(27-76)87-57)89-90(85,86)88-31(3)26-69-49(83)18-19-59(8)37(22-46(66)80)56-62(11)61(10,25-48(68)82)36(14-17-45(65)79)51(74-62)33(5)55-60(9,24-47(67)81)34(12-15-43(63)77)38(71-55)23-42-58(6,7)35(13-16-44(64)78)50(72-42)32(4)54(59)73-56;;/h20-21,23,28,31,34-37,41,52-53,56-57,76,84H,12-19,22,24-27H2,1-11H3,(H2,63,77)(H2,64,78)(H2,65,79)(H2,66,80)(H2,67,81)(H2,68,82)(H,69,83)(H,85,86);1H3;/q-1;;+4/p-1/b54-32-;; |
|---|
| InChI Key | GUIGZNJFVFPEGI-IGKGNRFDSA-M |
|---|
| Isomeric SMILES | C(CC(N)=O)C1C2=[N]3[Co+3]456(C)[N]7=CN(C8=CC(=C(C)C=C78)C)C7OC(C(C7O)OP([O-])(=O)OC(CNC(=O)CCC7(C(CC(N)=O)C(N4C7=C2C)C2(C(CC(N)=O)(C(CCC(N)=O)C(C(=C4[N]5=C(C(C4(CC(N)=O)C)CCC(N)=O)C=C3C1(C)C)C)=[N]62)C)C)C)C)CO |
|---|
| Average Molecular Weight | 1344.3823 |
|---|
| Monoisotopic Molecular Weight | 1343.587806391 |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as cobalamin derivatives. These are organic compounds containing a corrin ring, a cobalt atom, an a nucleotide moiety. Cobalamin Derivatives are actually derived from vitamin B12. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Tetrapyrroles and derivatives |
|---|
| Sub Class | Corrinoids |
|---|
| Direct Parent | Cobalamin derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Cobalamin
- Metallotetrapyrrole skeleton
- Pentose phosphate
- Benzimidazole
- Fatty amide
- Monosaccharide
- Organic phosphoric acid derivative
- Benzenoid
- Fatty acyl
- Pyrrolidine
- Pyrroline
- Tetrahydrofuran
- Carboxamide group
- Lactam
- Primary carboxylic acid amide
- Secondary alcohol
- Secondary carboxylic acid amide
- Organic transition metal salt
- Organic metal salt
- Azacycle
- Carboxylic acid amidine
- Carboxylic acid derivative
- Oxacycle
- Amidine
- Metalloheterocycle
- Organic salt
- Organic cobalt salt
- Organometallic compound
- Organic transition metal moeity
- Hydrocarbon derivative
- Organic oxide
- Transition metal alkyl
- Organonitrogen compound
- Organooxygen compound
- Organopnictogen compound
- Organic oxygen compound
- Primary alcohol
- Alcohol
- Carbonyl group
- Organic nitrogen compound
- Organic cation
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Physico-Chemical Properties - Experimental |
|---|
| Property | Value | Reference |
|---|
| Experimental logP | Not Available | |
|---|
| Experimental Water Solubility | Not Available | |
|---|
| Melting Point | Not Available | |
|---|
|
| Foods of Origin |
|---|
| Food | Content Range | Average | Reference |
|---|
| Food | | | Reference |
|---|
|
| Production Data |
|---|
| Production Method | commercial |
|---|
| Production Method Reference | Not Available |
|---|
| Production Method Reference File | Not Available |
|---|
| Quantity Available | Production upon request, up to 10 mg |
|---|
| Delivery Time | Not Available |
|---|
| Storage Form | solid |
|---|
| Storage Conditions | -80°C |
|---|
| Stability | Not Available |
|---|
| Purity | Not Available |
|---|
| Spectra |
|---|
| Spectral Data Upon Request | Not Available |
|---|
| Provider Information |
|---|
|
| Commercial Vendors |
|---|
| AKSci | H837 |
|---|
| AKSci | J90061 |
|---|
| Glentham | GV9033 |
|---|
| Toronto Research Chemicals | M202800 |
|---|