| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation date | 2015-10-09 22:33:27 UTC |
|---|
| Update date | 2017-01-19 02:36:41 UTC |
|---|
| FoodComEx ID | PC000928 |
|---|
| FoodDB Record | FDB020713 |
|---|
| Chemical Information |
|---|
| Name | Glycyrrhetic acid |
|---|
| Description | Aglycone from licorice (Glycyrrhiza glabra)
Glycyrrhetinic acid is a pentacyclic triterpenoid derivative of the beta-amyrin type obtained from the hydrolysis of glycyrrhizic acid, which was obtained from the herb liquorice. It is used in flavoring and it masks the bitter taste of drugs like aloe and quinine. Glycyrrhetic acid is found in tea and herbs and spices. |
|---|
| CAS Number | 471-53-4 |
|---|
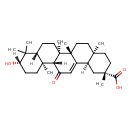
| Structure | |
|---|
| Synonyms | | Synonym | Source |
|---|
| α-Glycyrrhetinic acid | biospider | | 18-beta-Glycyrrhetic acid | biospider | | 18β-Glycyrrhetic acid | biospider | | 18β-Glycyrrhetinic acid | biospider | | 18β-Glycyrrhtinic acid | biospider | | 18b-Glycyrrhetic acid | biospider | | 18b-Glycyrrhetinic acid | biospider | | 18b-Glycyrrhtinic acid | biospider | | 18beta-Glycyrrhetic acid | biospider | | 18beta-Glycyrrhetinic acid | biospider | | 3-Glycyrrhetinic acid | HMDB | | 3-Hydroxy-11-oxoolean-12-en-29-Oate | HMDB | | 3-Hydroxy-11-oxoolean-12-en-29-Oic acid | HMDB | | 3-Hydroxy-11-oxoolean-12-en-29-Oic acid (acd/name 4.0) | HMDB | | 3b-Hydroxy-11-oxo-olean-12-en-30-Oate | HMDB | | 3b-Hydroxy-11-oxo-olean-12-en-30-Oic acid | HMDB | | 3b-Hydroxy-11-oxoolean-12-en-30-Oate | HMDB | | 3b-Hydroxy-11-oxoolean-12-en-30-Oic acid | HMDB | | 3beta-Hydroxy-11-oxo-18beta,20beta-olean-12-en-29-oic acid | biospider | | a-Glycyrrhetinic acid | db_source | | alpha-Glycyrrhetinic acid | biospider | | b-Glycyrrhetic acid | HMDB | | beta-Glycyrrhetic acid | HMDB | | Biogastrone acid | db_source | | Biosone | db_source | | Enoxolone | manual | | Glycyrrhetic acid | db_source | | Glycyrrhetin | db_source | | Glycyrrhetinate | biospider | | Glycyrrhetinic acid | db_source | | Glycyrrhitinic acid | biospider | | Rhetinic acid | db_source | | Uralenic acid | db_source |
|
|---|
| Chemical Formula | C30H46O4 |
|---|
| IUPAC name | (2S,4aS,6aS,6bR,8aR,10S,12aS,12bR,14bR)-10-hydroxy-2,4a,6a,6b,9,9,12a-heptamethyl-13-oxo-1,2,3,4,4a,5,6,6a,6b,7,8,8a,9,10,11,12,12a,12b,13,14b-icosahydropicene-2-carboxylic acid |
|---|
| InChI Identifier | InChI=1S/C30H46O4/c1-25(2)21-8-11-30(7)23(28(21,5)10-9-22(25)32)20(31)16-18-19-17-27(4,24(33)34)13-12-26(19,3)14-15-29(18,30)6/h16,19,21-23,32H,8-15,17H2,1-7H3,(H,33,34)/t19-,21-,22-,23+,26+,27-,28-,29+,30+/m0/s1 |
|---|
| InChI Key | MPDGHEJMBKOTSU-YKLVYJNSSA-N |
|---|
| Isomeric SMILES | [H][C@@]12C[C@](C)(CC[C@]1(C)CC[C@]1(C)C2=CC(=O)[C@]2([H])[C@@]3(C)CC[C@H](O)C(C)(C)[C@]3([H])CC[C@@]12C)C(O)=O |
|---|
| Average Molecular Weight | 470.694 |
|---|
| Monoisotopic Molecular Weight | 470.339609961 |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as triterpenoids. These are terpene molecules containing six isoprene units. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Prenol lipids |
|---|
| Sub Class | Triterpenoids |
|---|
| Direct Parent | Triterpenoids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Triterpenoid
- Cyclohexenone
- Cyclic alcohol
- Secondary alcohol
- Ketone
- Monocarboxylic acid or derivatives
- Carboxylic acid
- Carboxylic acid derivative
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Carbonyl group
- Alcohol
- Aliphatic homopolycyclic compound
|
|---|
| Molecular Framework | Aliphatic homopolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Physico-Chemical Properties - Experimental |
|---|
| Property | Value | Reference |
|---|
| Experimental logP | Not Available | |
|---|
| Experimental Water Solubility | Not Available | |
|---|
| Melting Point | Mp 300-304° | DFC |
|---|
|
| Foods of Origin |
|---|
| Food | Content Range | Average | Reference |
|---|
| Food | | | Reference |
|---|
|
| Production Data |
|---|
| Production Method | commercial |
|---|
| Production Method Reference | Not Available |
|---|
| Production Method Reference File | Not Available |
|---|
| Quantity Available | Production upon request, up to 3 g |
|---|
| Delivery Time | Not Available |
|---|
| Storage Form | solid |
|---|
| Storage Conditions | -80°C |
|---|
| Stability | Not Available |
|---|
| Purity | Not Available |
|---|
| Spectra |
|---|
| Spectral Data Upon Request | Not Available |
|---|
| Provider Information |
|---|
|
| Commercial Vendors |
|---|
| AKSci | C425 |
|---|
| AKSci | J10770 |
|---|
| Cayman Chemical | 11845 |
|---|
| Glentham | GP8041 |
|---|
| Toronto Research Chemicals | G735000 |
|---|