| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation date | 2015-10-09 22:32:33 UTC |
|---|
| Update date | 2017-01-19 02:36:35 UTC |
|---|
| FoodComEx ID | PC000760 |
|---|
| FoodDB Record | FDB022608 |
|---|
| Chemical Information |
|---|
| Name | Citicoline |
|---|
| Description | Citicoline, also known as CDP-colina or nicholin, belongs to the class of organic compounds known as pyrimidine ribonucleoside diphosphates. These are pyrimidine ribonucleotides with diphosphate group linked to the ribose moiety. Citicoline has also been shown to be able to inhibit mechanisms of apoptosis associated to cerebral ischemia and in certain neurodegeneration models, and to potentiate neuroplasticity mechanisms. Citicoline is a drug. Citicoline is an extremely weak basic (essentially neutral) compound (based on its pKa). Citicoline is an essential intermediate in the biosynthetic pathway of structural phospholipids in cell membranes, particularly phosphatidylcholine. Citicoline exists in all eukaryotes, ranging from yeast to humans. In humans, citicoline is involved in phospholipid biosynthesis. Outside of the human body, Citicoline has been detected, but not quantified in, several different foods, such as mountain yams, oil-seed camellia, rowals, mango, and pineappple sages. This could make citicoline a potential biomarker for the consumption of these foods. Citicoline is a safe drug, as shown by the toxicological tests conducted, that has no significant systemic cholinergic effects and is a well tolerated product. Thus, citicoline has been experimentally shown to increase norepinephrine and dopamine levels in the CNS. Citicoline activates biosynthesis of structural phospholipids of neuronal membranes, increases brain metabolism, and acts upon the levels of different neurotransmitters. Once absorbed, citicoline is widely distributed throughout the body, crosses the blood-brain barrier and reaches the central nervous system (CNS), where it is incorporated into the membrane and microsomal phospholipid fraction. In addition, citicoline has been shown to restore the activity of mitochondrial ATPase and membrane Na+/K+ATPase, to inhibit activation of certain phospholipases, and to accelerate reabsorption of cerebral edema in various experimental models. Owing to these pharmacological mechanisms, citicoline has a neuroprotective effect in hypoxic and ischemic conditions, decreasing the volume of ischemic lesion, and also improves learning and memory performance in animal models of brain aging. |
|---|
| CAS Number | 987-78-0 |
|---|
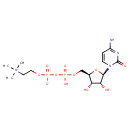
| Structure | |
|---|
| Synonyms | | Synonym | Source |
|---|
| [2-CYTIDYLATE-o'-onyloxyl]-ethyl-trimethyl-ammonium | ChEBI | | [2-CYTIDYLic acid-o'-onyloxyl]-ethyl-trimethyl-ammonium | Generator | | Audes | hmdb | | CDP-choline | hmdb | | CDP-Colina | ChEBI | | Cereb | hmdb | | Choline 5'-cytidine diate | HMDB | | Choline 5'-cytidine diphosphate | hmdb | | Choline cytidine diate | HMDB | | Choline cytidine diphosphate | hmdb | | Citicholine | hmdb | | Citicolina | ChEBI | | Citicoline | hmdb | | Citicolinum | ChEBI | | Citidin difosfato de colina | ChEBI | | Citidoline | hmdb | | Citifar | hmdb | | Colite | hmdb | | Corenalin | hmdb | | Cyscholin | hmdb | | Cytidindiocholin | ChEBI | | Cytidine 5-diate-trihydrogen | HMDB | | Cytidine 5-diphosphate-trihydrogen | hmdb | | Cytidine 5'-(choline diate) | ChEBI | | Cytidine 5'-(choline diic acid) | Generator | | Cytidine 5'-(choline diphosphate) | hmdb | | Cytidine 5'-(cholinyl pyroate) | ChEBI | | Cytidine 5'-(cholinyl pyroic acid) | Generator | | Cytidine 5'-(cholinyl pyrophosphate) | hmdb | | Cytidine 5'-diate choline | HMDB | | Cytidine 5'-diocholine | ChEBI | | Cytidine 5'-dioric choline | ChEBI | | Cytidine 5'-diphosphate choline | hmdb | | Cytidine 5'-diphosphocholine | hmdb | | Cytidine choline diate | HMDB | | Cytidine choline diphosphate | hmdb | | Cytidine diate choline | HMDB | | Cytidine diate choline ester | HMDB | | Cytidine diocholine | HMDB | | Cytidine diorylcholine | HMDB | | Cytidine diphosphate choline | hmdb | | Cytidine diphosphate choline ester | hmdb | | Cytidine diphosphocholine | hmdb | | Cytidine diphosphorylcholine | hmdb | | Cytidine-5' diocholine | HMDB | | cytidine-5' diphosphocholine | hmdb | | Cytidine-5'-pyroate-hydroxycholine | HMDB | | cytidine-5'-pyrophosphate-hydroxycholine | hmdb | | Cytidoline | hmdb | | Difosfocin | hmdb | | Emicholine F | hmdb | | Ensign | hmdb | | Haocolin | hmdb | | Hornbest | hmdb | | Neucolis | hmdb | | Nicholin | hmdb | | Nicolin | hmdb | | Niticolin | hmdb | | P-hydroxide[2-(trimethylammonio)ethyl] ester | hmdb | | Reagin | hmdb | | Recofnan | hmdb | | Recognan | hmdb | | Rexort | hmdb | | Sintoclar | hmdb | | Somazina | hmdb | | Somazine | hmdb | | Suncholin | hmdb |
|
|---|
| Chemical Formula | C14H26N4O11P2 |
|---|
| IUPAC name | {2-[({[(2R,3S,4R,5R)-5-(4-amino-2-oxo-1,2-dihydropyrimidin-1-yl)-3,4-dihydroxyoxolan-2-yl]methoxy}(hydroxy)phosphoryl phosphono)oxy]ethyl}trimethylazanium |
|---|
| InChI Identifier | InChI=1S/C14H26N4O11P2/c1-18(2,3)6-7-26-30(22,23)29-31(24,25)27-8-9-11(19)12(20)13(28-9)17-5-4-10(15)16-14(17)21/h4-5,9,11-13,19-20H,6-8H2,1-3H3,(H3-,15,16,21,22,23,24,25)/t9-,11-,12-,13-/m1/s1 |
|---|
| InChI Key | RZZPDXZPRHQOCG-OJAKKHQRSA-N |
|---|
| Isomeric SMILES | C[N+](C)(C)CCOP([O-])(=O)OP(O)(=O)OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)N1C=CC(N)=NC1=O |
|---|
| Average Molecular Weight | 488.324 |
|---|
| Monoisotopic Molecular Weight | 488.107330718 |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as pyrimidine ribonucleoside diphosphates. These are pyrimidine ribonucleotides with diphosphate group linked to the ribose moiety. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Nucleosides, nucleotides, and analogues |
|---|
| Class | Pyrimidine nucleotides |
|---|
| Sub Class | Pyrimidine ribonucleotides |
|---|
| Direct Parent | Pyrimidine ribonucleoside diphosphates |
|---|
| Alternative Parents | |
|---|
| Substituents | - Pyrimidine ribonucleoside diphosphate
- Pentose phosphate
- Pentose-5-phosphate
- Glycosyl compound
- N-glycosyl compound
- Phosphocholine
- Monosaccharide phosphate
- Organic pyrophosphate
- Pentose monosaccharide
- Aminopyrimidine
- Pyrimidone
- Monoalkyl phosphate
- Hydropyrimidine
- Monosaccharide
- Organic phosphoric acid derivative
- Phosphoric acid ester
- Pyrimidine
- Imidolactam
- Alkyl phosphate
- Tetrahydrofuran
- Quaternary ammonium salt
- Tetraalkylammonium salt
- Heteroaromatic compound
- Secondary alcohol
- 1,2-diol
- Organoheterocyclic compound
- Oxacycle
- Azacycle
- Organic nitrogen compound
- Organonitrogen compound
- Organopnictogen compound
- Amine
- Alcohol
- Organic oxide
- Organooxygen compound
- Organic oxygen compound
- Organic salt
- Hydrocarbon derivative
- Organic zwitterion
- Primary amine
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Physico-Chemical Properties - Experimental |
|---|
| Property | Value | Reference |
|---|
| Experimental logP | Not Available | |
|---|
| Experimental Water Solubility | Not Available | |
|---|
| Melting Point | Not Available | |
|---|
|
| Foods of Origin |
|---|
| Food | Content Range | Average | Reference |
|---|
| Food | | | Reference |
|---|
|
| Production Data |
|---|
| Production Method | commercial |
|---|
| Production Method Reference | Not Available |
|---|
| Production Method Reference File | Not Available |
|---|
| Quantity Available | Production upon request, up to 80 mg |
|---|
| Delivery Time | Not Available |
|---|
| Storage Form | solid |
|---|
| Storage Conditions | -80°C |
|---|
| Stability | Not Available |
|---|
| Purity | Not Available |
|---|
| Spectra |
|---|
| Spectral Data Upon Request | Not Available |
|---|
| Provider Information |
|---|
|
| Commercial Vendors |
|---|
| AKSci | K771 |
|---|