| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation date | 2015-10-09 22:32:18 UTC |
|---|
| Update date | 2017-01-19 02:36:34 UTC |
|---|
| FoodComEx ID | PC000736 |
|---|
| FoodDB Record | FDB020369 |
|---|
| Chemical Information |
|---|
| Name | Maltitoll |
|---|
| Description | Permitted bulk sweetener for foods
Commercially, maltitol is a disaccharide produced by Corn Products Specialty Ingredients (formerly SPI Polyols), Cargill, Roquette, and Towa, among other companies. Maltitol is made by hydrogenation of maltose obtained from starch. Its high sweetness allows it to be used without being mixed with other sweeteners, and exhibits negligible cooling effect (positive heat of solution) in comparison with other sugar alcohols, and is very similar to the subtle cooling effect of sucrose. It is used especially in production of sweets: sugarless hard candies, chewing gum, chocolates, baked goods, and ice cream. The pharmaceutical industry uses maltitol as an excipient where it is utilised as a low-calorie sweetening agent. Its similarity to sucrose allows it to be used in syrups with the advantage that crystallization (which may cause bottle caps to stick) is less likely. Maltitol may also be used as a plasticiser in gelatine capsules, as an emollient, and as a humectant.; In countries such as Australia, Norway and New Zealand, it carries a mandatory warning such as "Excessive consumption may have a laxative effect." In the United States, it is a Generally recognized as safe (GRAS) substance, with a recommendation of a warning about its laxative potential when consumed at levels of 100 grams per day or more.; Maltitol is a sugar alcohol (a polyol) used as a sugar substitute. It has 75-90% of the sweetness of sucrose (table sugar) and nearly identical properties, except for browning. It is used to replace table sugar because it has fewer calories, does not promote tooth decay and has a somewhat lesser effect on blood glucose. Chemically, maltitol is also known as 4-O-?-glucopyranosyl-D-sorbitol. Commercially, it is known under trade names such as Maltisorb and Maltisweet.; Maltitol is a sugar alcohol (polyol) used as a sugar substitute. It has 90% the sweetness of sugar and nearly identical properties, except for browning. It is used to very easily replace sugar and has less food energy, does not promote tooth decay and has a somewhat lower blood sugar response. Unfortunately, maltitol is well known to cause gastric distress, particularly if consumed in great quantities. Chemically, maltitol is also known as 4-O-alpha-Glucopyranosyl-D-sorbitol. Commercially, it is known under trade names such as Maltisorb and Maltisweet. Due to its slow absorption, excessive consumption of Maltitol can have laxative effect and often can cause gas and/or bloating. Maltitol is particularly demonized regarding gastric side effects because it is so easy for food producers to use it in vast quantities (due to its amazingly sugar-like properties) so consumers often end up consuming far more than they could most other sugar alcohols. While this is a major problem with maltitol, many sugar alcohols are far more likely to cause gastric distress than maltitol when compared gram-for-gram. -- Wikipedia |
|---|
| CAS Number | 585-88-6 |
|---|
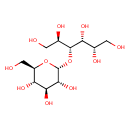
| Structure | |
|---|
| Synonyms | | Synonym | Source |
|---|
| (2S,3R,4R,5R)-4-{[(2R,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)tetrahydro-2H-pyran-2-yl]oxy}hexane-1,2,3,5,6-pentol | ChEBI | | 4-O-a-D-Glucopyranosyl-D-glucitol | Generator | | 4-O-alpha-D-Glucopyranosyl-D-glucitol | biospider | | 4-O-alpha-delta-Glucopyranosyl-delta-glucitol | biospider | | 4-O-α-D-glucopyranosyl-D-glucitol | Generator | | a-D-Glc-(1->4)-D-glc-ol | Generator | | a-D-Glcp-(1->4)-D-glc-ol | Generator | | a-D-Glucosyl-(1->4)-D-glucitol | Generator | | alpha-D-Glc-(1->4)-D-glc-ol | ChEBI | | alpha-D-Glcp-(1->4)-D-glc-ol | ChEBI | | alpha-D-Glucosyl-(1->4)-D-glucitol | ChEBI | | Amalti syrup | HMDB | | Amalty MR 100 | biospider | | D-4-O-alpha-D-Glucopyranosylglucitol | biospider | | D-Glucitol, 4-O-alpha-D-glucopyranosyl- | biospider | | D-glucopyranosyl-d-glucitol | biospider | | D-maltitol | biospider | | delta-4-O-alpha-delta-Glucopyranosylglucitol | biospider | | Delta-maltitol | biospider | | E965 | db_source | | Glucitol, 4-O-alpha-D-glucopyranosyl-, D- | biospider | | Malbit | biospider | | Malti MR | biospider | | Maltisorb | biospider | | Maltit | biospider | | Maltitol | db_source | | α-D-glc-(1->4)-D-glc-ol | Generator | | α-D-glcp-(1->4)-D-glc-ol | Generator | | α-D-glucosyl-(1->4)-D-glucitol | Generator |
|
|---|
| Chemical Formula | C12H24O11 |
|---|
| IUPAC name | (2S,3R,4R,5R)-4-{[(2R,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}hexane-1,2,3,5,6-pentol |
|---|
| InChI Identifier | InChI=1S/C12H24O11/c13-1-4(16)7(18)11(5(17)2-14)23-12-10(21)9(20)8(19)6(3-15)22-12/h4-21H,1-3H2 |
|---|
| InChI Key | VQHSOMBJVWLPSR-UHFFFAOYSA-N |
|---|
| Isomeric SMILES | OCC(O)C(O)C(OC1OC(CO)C(O)C(O)C1O)C(O)CO |
|---|
| Average Molecular Weight | 344.3124 |
|---|
| Monoisotopic Molecular Weight | 344.13186161 |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as fatty acyl glycosides of mono- and disaccharides. Fatty acyl glycosides of mono- and disaccharides are compounds composed of a mono- or disaccharide moiety linked to one hydroxyl group of a fatty alcohol or of a phosphorylated alcohol (phosphoprenols), a hydroxy fatty acid or to one carboxyl group of a fatty acid (ester linkage) or to an amino alcohol. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Fatty Acyls |
|---|
| Sub Class | Fatty acyl glycosides |
|---|
| Direct Parent | Fatty acyl glycosides of mono- and disaccharides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Fatty acyl glycoside of mono- or disaccharide
- Alkyl glycoside
- Disaccharide
- Glycosyl compound
- O-glycosyl compound
- Fatty alcohol
- Sugar alcohol
- Oxane
- Secondary alcohol
- Acetal
- Organoheterocyclic compound
- Oxacycle
- Polyol
- Organooxygen compound
- Primary alcohol
- Hydrocarbon derivative
- Organic oxygen compound
- Alcohol
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Physico-Chemical Properties - Experimental |
|---|
| Property | Value | Reference |
|---|
| Experimental logP | Not Available | |
|---|
| Experimental Water Solubility | Not Available | |
|---|
| Melting Point | Mp 149-152° | DFC |
|---|
|
| Foods of Origin |
|---|
| Food | Content Range | Average | Reference |
|---|
| Food | | | Reference |
|---|
|
| Production Data |
|---|
| Production Method | commercial |
|---|
| Production Method Reference | Not Available |
|---|
| Production Method Reference File | Not Available |
|---|
| Quantity Available | Production upon request, up to 1 g |
|---|
| Delivery Time | Not Available |
|---|
| Storage Form | solid |
|---|
| Storage Conditions | -80°C |
|---|
| Stability | Not Available |
|---|
| Purity | Not Available |
|---|
| Spectra |
|---|
| Spectral Data Upon Request | Not Available |
|---|
| Provider Information |
|---|
|
| Commercial Vendors |
|---|
| AKSci | 8789AJ |
|---|
| Fluka | HMDB0002928 |
|---|
| MetaSci | HMDB0002928 |
|---|
| Toronto Research Chemicals | M135010 |
|---|