| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation date | 2015-10-09 22:31:56 UTC |
|---|
| Update date | 2017-01-19 02:36:32 UTC |
|---|
| FoodComEx ID | PC000685 |
|---|
| FoodDB Record | FDB000893 |
|---|
| Chemical Information |
|---|
| Name | Phenol |
|---|
| Description | Phenol, also known as hydroxybenzene or carbolic acid, belongs to the class of organic compounds known as hydroxy benzenoids. Phenol is a toxic, colourless crystalline solid with a sweet tarry odor that resembles a hospital smell. It is commonly used as an antiseptic and disinfectant. It is active against a wide range of microorganisms including some fungi and viruses but is only slowly effective against spores. It has been used to disinfect skin and to relieve itching. Phenol is also used in the preparation of cosmetics including sunscreens, hair dyes, and skin lightening preparations. It is also used in the production of drugs (it is the starting material in the industrial production of aspirin), weedkillers, and synthetic resins. Phenol can be found in areas with high levels of motor traffic, therefore, people living in crowded urban areas are frequently exposed to traffic-derived phenol vapor. The average (mean +/- SD) phenol concentration in urine among normal individuals living in urban areas is 7.4 +/- 2.2 mg/g of creatinine. Exposure of the skin to concentrated phenol solutions causes chemical burns which may be severe. In laboratories where it is used, it is usually recommended that polyethylene glycol solution is kept available for washing off splashes. In some bacteria phenol can be directly synthesized from tyrosine via the enzyme tyrosine phenol-lyase [EC:4.1.99.2]. It can be produced by Escherichia and Pseudomonas. Phenol has been identified as a uremic toxin according to the European Uremic Toxin Working Group (PMID: 22626821). |
|---|
| CAS Number | 108-95-2 |
|---|
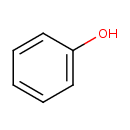
| Structure | |
|---|
| Synonyms | | Synonym | Source |
|---|
| Acide carbolique | ChEBI | | Acide phenique | ChEBI | | Anbesol | HMDB | | Benzenol | db_source | | Benzophenol | HMDB | | Campho-phenique cold sore gel | HMDB | | Campho-phenique gel | HMDB | | Campho-phenique liquid | HMDB | | Carbolate | Generator | | Carbolic acid | db_source | | Carbolic acid liquid | HMDB | | Carbolic oil | HMDB | | Carbolicum acidum | HMDB | | Carbolsaeure | ChEBI | | Carbolsaure | HMDB | | Cepastat lozenges | HMDB | | Cuticura pain relieving ointment | HMDB | | FEMA 3223 | db_source | | Fenol | HMDB | | Fenolo | HMDB | | Fenosmolin | HMDB | | Fenosmoline | HMDB | | Hydroxy-benzene | HMDB | | Hydroxybenzene | db_source | | IPH | HMDB | | IZAL | HMDB | | Karbolsaeure | ChEBI | | Liquefied phenol | HMDB | | Liquid phenol | HMDB | | Liquified phenol | HMDB | | Monohydroxy benzene | HMDB | | Monohydroxybenzene | HMDB | | Monophenol | HMDB | | Oxybenzene | ChEBI | | Paoscle | HMDB | | Phenate | Generator | | Phenic | HMDB | | Phenic acid | manual | | Phenic alcohol | HMDB | | Phenol alcohol | HMDB | | Phenol homopolymer | HMDB | | Phenol liquid | HMDB | | Phenol molten | HMDB | | Phenol polymer-bound | HMDB | | Phenol solution | HMDB | | Phenol synthetic | HMDB | | Phenolated water | HMDB | | Phenolated water for disinfection | HMDB | | Phenole | HMDB | | Phenosmolin | HMDB | | Phenyl alcohol | manual | | Phenyl hydrate | manual | | Phenyl hydroxide | manual | | Phenylate | Generator | | Phenylic acid | manual | | Phenylic alcohol | manual | | PHOH | ChEBI | | Synthetic phenol | HMDB | | Tea polyphenol | HMDB |
|
|---|
| Chemical Formula | C6H6O |
|---|
| IUPAC name | phenol |
|---|
| InChI Identifier | InChI=1S/C6H6O/c7-6-4-2-1-3-5-6/h1-5,7H |
|---|
| InChI Key | ISWSIDIOOBJBQZ-UHFFFAOYSA-N |
|---|
| Isomeric SMILES | OC1=CC=CC=C1 |
|---|
| Average Molecular Weight | 94.1112 |
|---|
| Monoisotopic Molecular Weight | 94.041864814 |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as 1-hydroxy-4-unsubstituted benzenoids. These are phenols that are unsubstituted at the 4-position. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Phenols |
|---|
| Sub Class | 1-hydroxy-4-unsubstituted benzenoids |
|---|
| Direct Parent | 1-hydroxy-4-unsubstituted benzenoids |
|---|
| Alternative Parents | |
|---|
| Substituents | - 1-hydroxy-4-unsubstituted benzenoid
- 1-hydroxy-2-unsubstituted benzenoid
- Monocyclic benzene moiety
- Organic oxygen compound
- Hydrocarbon derivative
- Organooxygen compound
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Physico-Chemical Properties - Experimental |
|---|
| Property | Value | Reference |
|---|
| Experimental logP | 1.46 | HANSCH,C ET AL. (1995) |
|---|
| Experimental Water Solubility | 82.8 mg/mL at 25 oC | SOUTHWORTH,GR & KELLER,JL (1986) |
|---|
| Melting Point | Fp 41° | DFC |
|---|
|
| Foods of Origin |
|---|
| Food | Content Range | Average | Reference |
|---|
| Food | | | Reference |
|---|
|
| Production Data |
|---|
| Production Method | commercial |
|---|
| Production Method Reference | Not Available |
|---|
| Production Method Reference File | Not Available |
|---|
| Quantity Available | Production upon request, up to 1 g |
|---|
| Delivery Time | Not Available |
|---|
| Storage Form | solid |
|---|
| Storage Conditions | -80°C |
|---|
| Stability | Not Available |
|---|
| Purity | Not Available |
|---|
| Spectra |
|---|
| Spectral Data Upon Request | Not Available |
|---|
| Provider Information |
|---|
|
| Commercial Vendors |
|---|
| Glentham | GK6990 |
|---|
| Glentham | GK0672 |
|---|
| MetaSci | HMDB0000228 |
|---|
| Sigma-Aldrich | HMDB0000228 |
|---|
| Toronto Research Chemicals | P318000 |
|---|
| Toronto Research Chemicals | P318005 |
|---|