| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation date | 2015-10-09 22:31:14 UTC |
|---|
| Update date | 2017-01-19 02:36:27 UTC |
|---|
| FoodComEx ID | PC000539 |
|---|
| FoodDB Record | FDB003277 |
|---|
| Chemical Information |
|---|
| Name | 2-Methylpropanoic acid |
|---|
| Description | Present in apple, morello cherry, guava fruit, wine grapes, pineapple, crispbread, other breads, cheeses, wines, scallop and several essential oils, e.g. Roman chamomile. Acid and simple esters used as flavouring agents
Isobutyric acid is a carboxylic or short chain fatty acid with characteristic sweat-like smell. Small amount of isobutyrate is generated via microbial (gut) metabolism. Small amounts may also be found in certain foods or fermented beverages. There is anosmia (genetic inability to smell) for the odor of isobutyric acid with a frequency of about 2.5%. (OMIM 207000). Isobutyric acid is slightly soluble in water but much more soluble in ethanol, ether and organic solvents. Isobutyric acid can affect people if breathed in and may be absorbed through the skin. Contact can irritate and burn the skin and eyes. Breathing Isobutyric acid can irritate the nose, throat and lungs causing coughing, wheezing and/or shortness of breath.; Isobutyric acid is an isomer of n-butyric acid; they have the same chemical formula C4H8 O2 but a different structure.; Isobutyric acid, also known as 2-methylpropanoic acid, is a carboxylic acid with structural formula (CH3)2-CH-COOH. It is found in the free state in carobs (Ceratonia siliqua) and in the root of Arnica dulcis, and as an ethyl ester in croton oil. |
|---|
| CAS Number | 79-31-2 |
|---|
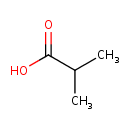
| Structure | |
|---|
| Synonyms | | Synonym | Source |
|---|
| α-methylpropanoic acid | biospider | | α-methylpropionic acid | biospider | | 1iup | biospider | | 2-METHYL-propionate | Generator | | 2-METHYL-PROPIONIC ACID | biospider | | 2-Methylpropanoate | biospider | | 2-Methylpropionate | biospider | | 2-Methylpropionic acid | biospider | | 2-Methylpropionsaeure | ChEBI | | 2,2-Dimethylacetate | Generator | | 2,2-Dimethylacetic acid | ChEBI | | 533-90-4 (calcium salt) | biospider | | a-Isobutyrate | Generator | | a-Isobutyric acid | Generator | | A-methylpropanoate | biospider | | A-methylpropanoic acid | biospider | | A-methylpropionate | biospider | | A-methylpropionic acid | biospider | | Acetic acid, dimethyl- | biospider | | alpha-Isobutyrate | Generator | | alpha-Isobutyric acid | ChEBI | | Alpha-methylpropanoate | biospider | | Alpha-methylpropanoic acid | biospider | | Alpha-methylpropionate | biospider | | Alpha-methylpropionic acid | biospider | | ALQ | biospider | | Cenex RP b2 | biospider | | Dimethylacetate | biospider | | Dimethylacetic acid | biospider | | FEMA 2222 | db_source | | I-butyrate | biospider | | I-butyric acid | biospider | | ISB | biospider | | Iso-butyrate | biospider | | Iso-butyric acid | biospider | | iso-C3H7COOH | biospider | | Isobutanoate | biospider | | Isobutanoic acid | biospider | | Isobuttersaeure | ChEBI | | Isobutyrate | biospider | | Isobutyric acid | biospider | | Isobutyric acid [UN2529] [Flammable liquid] | biospider | | Isobutyric acid, 8CI | db_source | | Isopropylformate | biospider | | Isopropylformic acid | biospider | | Methylpropanoic acid | biospider | | Methylpropanoic acid, 2- | biospider | | Methylpropionic acid | biospider | | Propanoic acid, 2-methyl- | biospider | | Propionic acid, 2-methyl- | biospider | | Tenox EBP 2 | biospider | | Tenox IBP-2 | biospider | | Tenox IBP-2 Grain Pr | biospider | | Tenox IBP-2 Grain Pr. | biospider | | α-isobutyrate | Generator | | α-isobutyric acid | Generator | | α-methylpropanoate | Generator | | α-methylpropanoic acid | Generator | | α-methylpropionate | Generator | | α-methylpropionic acid | Generator |
|
|---|
| Chemical Formula | C4H8O2 |
|---|
| IUPAC name | 2-methylpropanoic acid |
|---|
| InChI Identifier | InChI=1S/C4H8O2/c1-3(2)4(5)6/h3H,1-2H3,(H,5,6) |
|---|
| InChI Key | KQNPFQTWMSNSAP-UHFFFAOYSA-N |
|---|
| Isomeric SMILES | CC(C)C(O)=O |
|---|
| Average Molecular Weight | 88.1051 |
|---|
| Monoisotopic Molecular Weight | 88.0524295 |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as carboxylic acids. Carboxylic acids are compounds containing a carboxylic acid group with the formula -C(=O)OH. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Carboxylic acids |
|---|
| Direct Parent | Carboxylic acids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Monocarboxylic acid or derivatives
- Carboxylic acid
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Carbonyl group
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Physico-Chemical Properties - Experimental |
|---|
| Property | Value | Reference |
|---|
| Experimental logP | 0.94 | SANGSTER (1993) |
|---|
| Experimental Water Solubility | 167 mg/mL at 20 oC | YALKOWSKY,SH & DANNENFELSER,RM (1992) |
|---|
| Melting Point | Fp -47° | DFC |
|---|
|
| Foods of Origin |
|---|
| Food | Content Range | Average | Reference |
|---|
| Food | | | Reference |
|---|
|
| Production Data |
|---|
| Production Method | commercial |
|---|
| Production Method Reference | Not Available |
|---|
| Production Method Reference File | Not Available |
|---|
| Quantity Available | Production upon request, up to 4 g |
|---|
| Delivery Time | Not Available |
|---|
| Storage Form | liquid |
|---|
| Storage Conditions | -80°C |
|---|
| Stability | Not Available |
|---|
| Purity | Not Available |
|---|
| Spectra |
|---|
| Spectral Data Upon Request | Not Available |
|---|
| Provider Information |
|---|
|
| Commercial Vendors |
|---|
| AKSci | K400 |
|---|
| MetaSci | HMDB0001873 |
|---|
| Tokyo Chemical Industry | HMDB0001873 |
|---|
| Toronto Research Chemicals | I781005 |
|---|