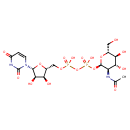
| (2R,3R,4R,5S,6R)-3-(acetylamino)-4,5-Dihydroxy-6-(hydroxymethyl)tetrahydro-2H-pyran-2-yl [(2R,3S,4R,5R)-5-(2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl)-3,4-dihydroxytetrahydrofuran-2-yl]methyl dihydrogen diate (non-preferred name) | ChEBI |
| (2R,3R,4R,5S,6R)-3-(acetylamino)-4,5-Dihydroxy-6-(hydroxymethyl)tetrahydro-2H-pyran-2-yl [(2R,3S,4R,5R)-5-(2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl)-3,4-dihydroxytetrahydrofuran-2-yl]methyl dihydrogen diic acid (non-preferred name) | Generator |
| [[3-acetylamino-4,5-Dihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy-hydroxy-oryl]oxy-[[5-(2,4-dioxopyrimidin-1-yl)-3,4-dihydroxy-oxolan-2-yl]methoxy]inate | HMDB |
| [[3-acetylamino-4,5-Dihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy-hydroxy-oryl]oxy-[[5-(2,4-dioxopyrimidin-1-yl)-3,4-dihydroxy-oxolan-2-yl]methoxy]inic acid | HMDB |
| [[3-acetylamino-4,5-dihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy-hydroxy-phosphoryl]oxy-[[5-(2,4-dioxopyrimidin-1-yl)-3,4-dihydroxy-oxolan-2-yl]methoxy]phosphinate | hmdb |
| [[3-acetylamino-4,5-dihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy-hydroxy-phosphoryl]oxy-[[5-(2,4-dioxopyrimidin-1-yl)-3,4-dihydroxy-oxolan-2-yl]methoxy]phosphinic acid | hmdb |
| N-[2-[[[5-[(2,4-dioxo-1H-Pyrimidin-1-yl)]-3,4-dihydroxy-tetrahydrofuran-2-yl]methoxy-hydroxy-inoyl]oxy-hydroxy-inoyl]oxy-4,5-dihydroxy-6-(hydroxymethyl)tetrahydropyran-3-yl]acetamide | HMDB |
| N-[2-[[[5-[(2,4-dioxo-1H-pyrimidin-1-yl)]-3,4-dihydroxy-tetrahydrofuran-2-yl]methoxy-hydroxy-phosphinoyl]oxy-hydroxy-phosphinoyl]oxy-4,5-dihydroxy-6-(hydroxymethyl)tetrahydropyran-3-yl]acetamide | hmdb |
| UDP-a-D-N-acetylglucosamine | hmdb |
| UDP-acetyl-D-glucosamine | hmdb |
| UDP-acetyl-delta-glucosamine | hmdb |
| UDP-acetylglucosamine | hmdb |
| UDP-alpha-D-N-acetylglucosamine | hmdb |
| UDP-alpha-delta-N-acetylglucosamine | hmdb |
| UDP-GlcNAc | hmdb |
| UDP-N-acetyl-D-glucosamine | hmdb |
| UDP-N-acetyl-delta-glucosamine | hmdb |
| UDP-N-acetyl-glucosamine | hmdb |
| UDP-N-acetylglucosamine | hmdb |
| UPPAG | hmdb |
| Uridine 5'-dio-N-acetlyglucosamine | HMDB |
| Uridine 5'-dio-N-acetylglucosamine | HMDB |
| Uridine 5'-Diphospho-N-Acetlyglucosamine | hmdb |
| Uridine 5'-diphospho-N-acetylglucosamine | hmdb |
| Uridine diate N-acetyl-D-glucosamine | HMDB |
| Uridine diate N-acetyl-delta-glucosamine | HMDB |
| Uridine diate N-acetylglucosamine | HMDB |
| Uridine dio-2-acetamido-2-deoxy-D-glucose | HMDB |
| Uridine dio-2-acetamido-2-deoxy-delta-glucose | HMDB |
| Uridine dio-N-acetyl-D-glucosamine | HMDB |
| Uridine dio-N-acetyl-delta-glucosamine | HMDB |
| Uridine dio-N-acetylglucosamine | HMDB |
| Uridine dioacetylglucosamine | HMDB |
| Uridine diphosphate N-acetyl-D-glucosamine | hmdb |
| Uridine diphosphate N-acetyl-delta-glucosamine | hmdb |
| Uridine diphosphate N-acetylglucosamine | hmdb |
| Uridine diphospho-2-acetamido-2-deoxy-D-glucose | hmdb |
| Uridine diphospho-2-acetamido-2-deoxy-delta-glucose | hmdb |
| Uridine diphospho-N-acetyl-D-glucosamine | hmdb |
| Uridine diphospho-N-acetyl-delta-glucosamine | hmdb |
| Uridine diphospho-N-acetylglucosamine | hmdb |
| uridine diphosphoacetylglucosamine | hmdb |
| Uridine pyroate 2-acetamido-2-deoxy-a-D-glucopyranosyl ester | HMDB |
| Uridine pyroate 2-acetamido-2-deoxy-alpha-D-glucopyranosyl ester | HMDB |
| Uridine pyroate 2-acetamido-2-deoxy-alpha-delta-glucopyranosyl ester | HMDB |
| Uridine pyrooacetylglucosamine | HMDB |
| Uridine pyrophosphate 2-acetamido-2-deoxy-a-D-glucopyranosyl ester | hmdb |
| Uridine pyrophosphate 2-acetamido-2-deoxy-alpha-D-glucopyranosyl ester | hmdb |
| Uridine pyrophosphate 2-acetamido-2-deoxy-alpha-delta-glucopyranosyl ester | hmdb |
| Uridine pyrophosphoacetylglucosamine | hmdb |
| URIDINE-diATE-N-acetylglucosamine | ChEBI |
| URIDINE-diic acid-N-acetylglucosamine | Generator |