| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation date | 2015-10-09 22:29:20 UTC |
|---|
| Update date | 2017-01-19 02:36:17 UTC |
|---|
| FoodComEx ID | PC000228 |
|---|
| FoodDB Record | FDB021806 |
|---|
| Chemical Information |
|---|
| Name | Adenosine monophosphate |
|---|
| Description | Adenosine monophosphate, also known as 5'-adenylic acid and abbreviated AMP, is a nucleotide that is found in RNA. It is an ester of phosphoric acid with the nucleoside adenosine. AMP consists of the phosphate group, the pentose sugar ribose, and the nucleobase adenine. AMP can be produced during ATP synthesis by the enzyme adenylate kinase. AMP has recently been approved as a 'Bitter Blocker' additive to foodstuffs. When AMP is added to bitter foods or foods with a bitter aftertaste it makes them seem 'sweeter'. This potentially makes lower calorie food products more palatable [HMDB] |
|---|
| CAS Number | 61-19-8 |
|---|
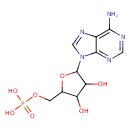
| Structure | |
|---|
| Synonyms | | Synonym | Source |
|---|
| 5'-Adenosine monoate | ChEBI | | 5'-Adenosine monoic acid | Generator | | 5'-Adenylate | Generator | | 5'-Adenylic acid | ChEBI | | 5'-AMP | ChEBI | | 5'-O-Onoadenosine | ChEBI | | Aden | HMDB | | Adenosine 5'-(dihydrogen ate) | ChEBI | | Adenosine 5'-(dihydrogen ic acid) | Generator | | Adenosine 5'-ate | ChEBI | | Adenosine 5'-ic acid | Generator | | Adenosine 5'-monoate | ChEBI | | Adenosine 5'-monoic acid | Generator | | Adenosine 5'-orate | HMDB | | Adenosine 5'-oric acid | HMDB | | Adenosine ate | ChEBI | | Adenosine ic acid | Generator | | ADENOSINE monoATE | ChEBI | | ADENOSINE monoic acid | Generator | | Adenosine-5-monoorate | HMDB | | Adenosine-5-monooric acid | HMDB | | Adenosine-5'-monoate | Generator | | Adenosine-5'-monoic acid | Generator | | Adenosine-5'-monoorate | HMDB | | Adenosine-5'-monooric acid | ChEBI | | Adenosine-5'p | ChEBI | | Adenosine-ate | HMDB | | Adenosine-monoate | HMDB | | Adenosini as | ChEBI | | Adenovite | HMDB | | Adenylate | ChEBI | | Adenylic acid | ChEBI | | Ado5'p | ChEBI | | ate D'adenosine | ChEBI | | Cardiomone | HMDB | | Entaside | HMDB | | Fosfato de adenosina | ChEBI | | ic acid D'adenosine | Generator | | Lycedan | HMDB | | Muscle adenylate | HMDB | | Muscle adenylic acid | HMDB | | My-b-den | HMDB | | My-beta-den | HMDB | | PAdo | ChEBI | | Phosaden | HMDB |
|
|---|
| Chemical Formula | C10H14N5O7P |
|---|
| IUPAC name | {[5-(6-amino-9H-purin-9-yl)-3,4-dihydroxyoxolan-2-yl]methoxy}phosphonic acid |
|---|
| InChI Identifier | InChI=1S/C10H14N5O7P/c11-8-5-9(13-2-12-8)15(3-14-5)10-7(17)6(16)4(22-10)1-21-23(18,19)20/h2-4,6-7,10,16-17H,1H2,(H2,11,12,13)(H2,18,19,20) |
|---|
| InChI Key | UDMBCSSLTHHNCD-UHFFFAOYSA-N |
|---|
| Isomeric SMILES | NC1=C2N=CN(C3OC(COP(O)(O)=O)C(O)C3O)C2=NC=N1 |
|---|
| Average Molecular Weight | 347.2212 |
|---|
| Monoisotopic Molecular Weight | 347.063084339 |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as purine ribonucleoside monophosphates. These are nucleotides consisting of a purine base linked to a ribose to which one monophosphate group is attached. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Nucleosides, nucleotides, and analogues |
|---|
| Class | Purine nucleotides |
|---|
| Sub Class | Purine ribonucleotides |
|---|
| Direct Parent | Purine ribonucleoside monophosphates |
|---|
| Alternative Parents | |
|---|
| Substituents | - Purine ribonucleoside monophosphate
- Pentose phosphate
- Pentose-5-phosphate
- Glycosyl compound
- N-glycosyl compound
- 6-aminopurine
- Monosaccharide phosphate
- Pentose monosaccharide
- Imidazopyrimidine
- Purine
- Aminopyrimidine
- Monoalkyl phosphate
- Alkyl phosphate
- Monosaccharide
- Pyrimidine
- Imidolactam
- N-substituted imidazole
- Organic phosphoric acid derivative
- Phosphoric acid ester
- Tetrahydrofuran
- Azole
- Imidazole
- Heteroaromatic compound
- Secondary alcohol
- 1,2-diol
- Organoheterocyclic compound
- Oxacycle
- Azacycle
- Organonitrogen compound
- Primary amine
- Hydrocarbon derivative
- Organic oxide
- Alcohol
- Organopnictogen compound
- Organic oxygen compound
- Organic nitrogen compound
- Organooxygen compound
- Amine
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Physico-Chemical Properties - Experimental |
|---|
| Property | Value | Reference |
|---|
| Experimental logP | Not Available | |
|---|
| Experimental Water Solubility | Not Available | |
|---|
| Melting Point | Not Available | |
|---|
|
| Foods of Origin |
|---|
| Food | Content Range | Average | Reference |
|---|
| Food | | | Reference |
|---|
|
| Production Data |
|---|
| Production Method | commercial |
|---|
| Production Method Reference | Not Available |
|---|
| Production Method Reference File | Not Available |
|---|
| Quantity Available | Production upon request, up to 2 g |
|---|
| Delivery Time | Not Available |
|---|
| Storage Form | solid |
|---|
| Storage Conditions | -80°C |
|---|
| Stability | Not Available |
|---|
| Purity | Not Available |
|---|
| Spectra |
|---|
| Spectral Data Upon Request | Not Available |
|---|
| Provider Information |
|---|
|
| Commercial Vendors |
|---|
| AKSci | C904 |
|---|
| AKSci | J93669 |
|---|
| Toronto Research Chemicals | A281780 |
|---|