| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation date | 2015-10-09 22:28:56 UTC |
|---|
| Update date | 2017-01-19 02:36:15 UTC |
|---|
| FoodComEx ID | PC000161 |
|---|
| FoodDB Record | FDB012804 |
|---|
| Chemical Information |
|---|
| Name | Formic acid |
|---|
| Description | Formic acid, also known as methanoic acid, is the simplest carboxylic acid, and has the chemical formula HCOOH. It is an important intermediate in chemical synthesis and occurs naturally, most notably in some ants. Esters, salts, and the anion derived from formic acid are called formates. Industrially, formic acid is produced through the oxidation of methanol. Formate is an intermediate in normal metabolism. It takes part in the metabolism of one-carbon compounds and its carbon may appear in methyl groups undergoing transmethylation. It is eventually oxidized to carbon dioxide. Formate is typically produced as a by-product in the production of acetate. It is responsible for both metabolic acidosis and disrupting mitochondrial electron transport and energy production by inhibiting cytochrome oxidase activity, the terminal electron acceptor of the electron transport chain. Cell death from cytochrome oxidase inhibition by formate is believed to result partly from depletion of ATP, reducing energy concentrations so that essential cell functions cannot be maintained. Furthermore, inhibition of cytochrome oxidase by formate may also cause cell death by increased production of cytotoxic reactive oxygen species (ROS) secondary to the blockade of the electron transport chain. In nature, formic acid is found in the stings and bites of many insects of the order Hymenoptera, including bees and ants. The principal use of formic acid is as a preservative and antibacterial agent in livestock feed. When sprayed on fresh hay or other silage, it arrests certain decay processes and causes the feed to retain its nutritive value longer. Urinary formate is produced by Escherichia coli, Pseudomonas aeruginosa, Klebsiella pneumonia, Enterobacter, Acinetobacter, Proteus mirabilis, Citrobacter frundii, Enterococcus faecalis, Streptococcus group B, Staphylococcus saprophyticus (PMID: 22292465). |
|---|
| CAS Number | 64-18-6 |
|---|
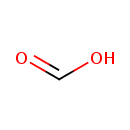
| Structure | |
|---|
| Synonyms | | Synonym | Source |
|---|
| Acide formique | ChEBI | | Add-F | HMDB | | Amasil | biospider | | Ameisensaeure | ChEBI | | Ameisensaure | HMDB | | Aminate | biospider | | Aminic acid | biospider | | Bilorin | biospider | | Collo-bueglatt | biospider | | Collo-didax | biospider | | FEMA 2487 | db_source | | Formate | biospider | | Formira | biospider | | Formisoton | biospider | | Formylate | biospider | | Formylic acid | biospider | | H-COOH | ChEBI | | HCO2H | ChEBI | | HCOOH | ChEBI | | Hydrogen carboxylate | biospider | | Hydrogen carboxylic acid | biospider | | Methanoate | biospider | | Methanoic acid | db_source | | Methanoic acid monomer | HMDB | | Methoate | Generator | | Methoic acid | biospider | | Myrmicyl | HMDB | | Sodium formate | HMDB | | Sybest | biospider | | Wonderbond hardener m 600L | HMDB |
|
|---|
| Chemical Formula | CH2O2 |
|---|
| IUPAC name | formic acid |
|---|
| InChI Identifier | InChI=1S/CH2O2/c2-1-3/h1H,(H,2,3) |
|---|
| InChI Key | BDAGIHXWWSANSR-UHFFFAOYSA-N |
|---|
| Isomeric SMILES | OC=O |
|---|
| Average Molecular Weight | 46.0254 |
|---|
| Monoisotopic Molecular Weight | 46.005479308 |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as carboxylic acids. Carboxylic acids are compounds containing a carboxylic acid group with the formula -C(=O)OH. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Carboxylic acids |
|---|
| Direct Parent | Carboxylic acids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Monocarboxylic acid or derivatives
- Carboxylic acid
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Carbonyl group
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Physico-Chemical Properties - Experimental |
|---|
| Property | Value | Reference |
|---|
| Experimental logP | -0.54 | HANSCH,C ET AL. (1995) |
|---|
| Experimental Water Solubility | 1000 mg/mL at 25 oC | RIDDICK,JA et al. (1986) |
|---|
| Melting Point | Mp 8.4° | DFC |
|---|
|
| Foods of Origin |
|---|
| Food | Content Range | Average | Reference |
|---|
| Food | | | Reference |
|---|
|
| Production Data |
|---|
| Production Method | commercial |
|---|
| Production Method Reference | Not Available |
|---|
| Production Method Reference File | Not Available |
|---|
| Quantity Available | Production upon request, up to 500 mg |
|---|
| Delivery Time | Not Available |
|---|
| Storage Form | liquid |
|---|
| Storage Conditions | -80°C |
|---|
| Stability | Not Available |
|---|
| Purity | Not Available |
|---|
| Spectra |
|---|
| Spectral Data Upon Request | Not Available |
|---|
| Provider Information |
|---|
|
| Commercial Vendors |
|---|
| AKSci | Y1306 |
|---|
| Glentham | GK4394 |
|---|
| Toronto Research Chemicals | F692900 |
|---|