| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation date | 2015-10-09 22:28:48 UTC |
|---|
| Update date | 2017-01-19 02:36:14 UTC |
|---|
| FoodComEx ID | PC000145 |
|---|
| FoodDB Record | FDB012362 |
|---|
| Chemical Information |
|---|
| Name | beta-Sitosterol |
|---|
| Description | beta-Sitosterol is one of several phytosterols (plant sterols) with chemical structures similar to that of cholesterol. Sitosterols are white, waxy powders with a characteristic odor. They are hydrophobic and soluble in alcohols. beta-Sitosterol is found in many foods, some of which are ginseng, globe artichoke, sesbania flower, and common oregano. |
|---|
| CAS Number | 83-46-5 |
|---|
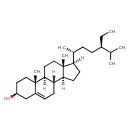
| Structure | |
|---|
| Synonyms | | Synonym | Source |
|---|
| (-)-b-Sitosterol | biospider | | (-)-beta-Sitosterol | biospider | | (-)-β-sitosterol | Generator | | (24R)-Ethylcholest-5-en-3b-ol | biospider | | (24R)-Ethylcholest-5-en-3beta-ol | biospider | | (24R)-Ethylcholest-5-en-3β-ol | Generator | | (24R)-Stigmast-5-en-3b-ol | biospider | | (24R)-Stigmast-5-en-3beta-ol | biospider | | (24R)-Stigmast-5-en-3β-ol | Generator | | (3b)-Stigmast-5-en-3-ol | Generator | | (3beta)-Stigmast-5-en-3-ol | ChEBI | | (3β)-stigmast-5-en-3-ol | Generator | | α-Dihydrofucosterol | biospider | | α-Ethylcholesterol | biospider | | β-Sitosterin | biospider | | 22,23-dihydro-Stigmasterol | HMDB | | 22,23-Dihydrostigmasterol | db_source | | 24-alpha-Ethylcholesterol | biospider | | 24α-Ethylcholesterol | biospider | | 24a-Ethylcholesterol | biospider | | 24alpha-Ethylcholesterol | ChEBI | | 24α-ethylcholesterol | Generator | | a-Dihydrofucosterol | Generator | | a-Phytosterol | HMDB | | alpha-Dihydrofucosterol | biospider | | alpha-Phytosterol | biospider | | Angelicin (steroid) | manual | | b-Sitosterin | biospider | | b-Sitosterol | db_source | | beta-Phytosterol | HMDB | | Beta-Sitosterin | biospider | | Beta-Sitosterol | biospider | | Cinchol | db_source | | Cupreol | db_source | | D5-Stigmasten-3b-ol | biospider | | delta5-Stigmasten-3-beta-ol | biospider | | delta5-Stigmasten-3b-ol | biospider | | Harzol | biospider | | Nimbosterol | db_source | | Papaveristerol | db_source | | Phytosterol | HMDB | | Prostasal | biospider | | Quebrachol | db_source | | Raphanisterol | db_source | | Rhamnol | db_source | | Sito-lande | biospider | | Sitosterol | db_source | | Sitosterol, β | biospider | | Slanutosterol | db_source | | Sobatum | HMDB | | Stigmast-5-en-3-beta-ol | biospider | | Stigmast-5-en-3-ol, (3β)- | biospider | | Stigmast-5-en-3-ol, (3beta)- | biospider | | Stigmast-5-en-3β-ol | biospider | | Stigmast-5-en-3b-ol | HMDB | | Stigmast-5-en-3beta-ol | biospider | | Stigmasterol, 22,23-dihydro- | biospider | | Triastonal | ChEBI | | Verosterol | db_source | | α-dihydrofucosterol | Generator | | β-sitosterin | Generator | | β-sitosterol | Generator |
|
|---|
| Chemical Formula | C29H50O |
|---|
| IUPAC name | (1S,2R,5S,10S,11S,14R,15R)-14-[(2R,5R)-5-ethyl-6-methylheptan-2-yl]-2,15-dimethyltetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadec-7-en-5-ol |
|---|
| InChI Identifier | InChI=1S/C29H50O/c1-7-21(19(2)3)9-8-20(4)25-12-13-26-24-11-10-22-18-23(30)14-16-28(22,5)27(24)15-17-29(25,26)6/h10,19-21,23-27,30H,7-9,11-18H2,1-6H3/t20-,21-,23+,24+,25-,26+,27+,28+,29-/m1/s1 |
|---|
| InChI Key | KZJWDPNRJALLNS-VJSFXXLFSA-N |
|---|
| Isomeric SMILES | [H][C@@]1(CC[C@@]2([H])[C@]3([H])CC=C4C[C@@H](O)CC[C@]4(C)[C@@]3([H])CC[C@]12C)[C@H](C)CC[C@@H](CC)C(C)C |
|---|
| Average Molecular Weight | 414.718 |
|---|
| Monoisotopic Molecular Weight | 414.38616623 |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as stigmastanes and derivatives. These are sterol lipids with a structure based on the stigmastane skeleton, which consists of a cholestane moiety bearing an ethyl group at the carbon atom C24. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Steroids and steroid derivatives |
|---|
| Sub Class | Stigmastanes and derivatives |
|---|
| Direct Parent | Stigmastanes and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - C24-propyl-sterol-skeleton
- Stigmastane-skeleton
- Triterpenoid
- 3-hydroxy-delta-5-steroid
- 3-hydroxysteroid
- Hydroxysteroid
- 3-beta-hydroxysteroid
- 3-beta-hydroxy-delta-5-steroid
- Delta-5-steroid
- Cyclic alcohol
- Secondary alcohol
- Alcohol
- Organooxygen compound
- Hydrocarbon derivative
- Organic oxygen compound
- Aliphatic homopolycyclic compound
|
|---|
| Molecular Framework | Aliphatic homopolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Physico-Chemical Properties - Experimental |
|---|
| Property | Value | Reference |
|---|
| Experimental logP | Not Available | |
|---|
| Experimental Water Solubility | Not Available | |
|---|
| Melting Point | Mp 136-137° | DFC |
|---|
|
| Foods of Origin |
|---|
| Food | Content Range | Average | Reference |
|---|
| Food | | | Reference |
|---|
|
| Production Data |
|---|
| Production Method | commercial |
|---|
| Production Method Reference | Not Available |
|---|
| Production Method Reference File | Not Available |
|---|
| Quantity Available | Production upon request, up to 2 g |
|---|
| Delivery Time | Not Available |
|---|
| Storage Form | solid |
|---|
| Storage Conditions | -80°C |
|---|
| Stability | Not Available |
|---|
| Purity | Not Available |
|---|
| Spectra |
|---|
| Spectral Data Upon Request | Not Available |
|---|
| Provider Information |
|---|
|
| Commercial Vendors |
|---|
| AKSci | 0140AP |
|---|
| AKSci | M047 |
|---|
| Cayman Chemical | 11756 |
|---|
| Glentham | GP9580 |
|---|
| Toronto Research Chemicals | S497050 |
|---|