| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation date | 2015-10-09 22:28:22 UTC |
|---|
| Update date | 2017-01-19 02:36:12 UTC |
|---|
| FoodComEx ID | PC000103 |
|---|
| FoodDB Record | FDB000556 |
|---|
| Chemical Information |
|---|
| Name | L-Alanine |
|---|
| Description | L-Alanine or Alanine, abbreviated Ala or A, is a non-essential amino acid made in the body from either the conversion of the carbohydrate pyruvate or the breakdown of DNA and the dipeptides carnosine and anserine. Normal alanine metabolism, like that of other amino acids, is highly dependent upon enzymes that contain vitamin B6. Alanine is an important participant as well as a regulator of glucose metabolism. Alanine levels parallel blood sugar levels in both diabetes and hypoglycemia, and alanine reduces both severe hypoglycemia and the ketosis of diabetes. It is highly concentrated in muscle and is one of the most important amino acids released by muscle, functioning as a major energy source. Plasma alanine is often decreased when the BCAA (branched-chain amino acids) are deficient. This finding may relate to muscle metabolism. It is an important amino acid for lymphocyte reproduction and immunity. Alanine therapy has helped dissolve kidney stones in experimental animals. L-Alanine has been found to be associated with glucagon deficiency, which is an inborn error of metabolism. Alanine is highly concentrated in meat products and other high-protein foods like wheat germ and cottage cheese. |
|---|
| CAS Number | 56-41-7 |
|---|
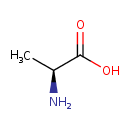
| Structure | |
|---|
| Synonyms | | Synonym | Source |
|---|
| (2S)-2-Aminopropanoate | biospider | | (2S)-2-Aminopropanoic acid | biospider | | (L)-Alanine | biospider | | (S)-(+)-Alanine | biospider | | (S)-2-Amino-propanoate | biospider | | (S)-2-Amino-propanoic acid | biospider | | (S)-2-Aminopropanoate | biospider | | (S)-2-Aminopropanoic acid | biospider | | (S)-2-Aminopropionic acid | biospider | | (S)-Alanine | biospider | | α-Aminopropionic acid | biospider | | 2-Aminopropanoate | HMDB | | 2-Aminopropanoic acid | HMDB | | 2-Aminopropanoic acid, L- | biospider | | 2-Aminopropionate | HMDB | | 2-Aminopropionic acid | HMDB | | 2-Ammoniopropanoate | HMDB | | 2-Ammoniopropanoic acid | HMDB | | A | ChEBI | | a-Alanine | HMDB | | a-Aminopropionate | HMDB | | a-Aminopropionic acid | HMDB | | ALA | biospider | | Alanine | biospider | | Alanine, INN, USAN; L-form | db_source | | Alanine, L- (7CI,8CI) | biospider | | alpha-Alanine | biospider | | alpha-Aminopropanoate | biospider | | alpha-Aminopropanoic acid | biospider | | alpha-Aminopropionate | biospider | | alpha-Aminopropionic acid | biospider | | L-(+)-Alanine | HMDB | | L-&alpha-Alanine | biospider | | L-α-Alanine | biospider | | L-α-Aminopropionic acid | biospider | | L-2-Aminopropanoate | biospider | | L-2-Aminopropanoic acid | biospider | | L-2-Aminopropionate | biospider | | L-2-Aminopropionic acid | biospider | | L-a-Alanine | biospider | | L-a-Aminopropionate | biospider | | L-a-Aminopropionic acid | biospider | | L-Alanin | ChEBI | | L-Alanine (9CI) | biospider | | L-alpha-Alanine | biospider | | L-alpha-Aminopropionate | biospider | | L-alpha-Aminopropionic acid | biospider | | L-S-Aminopropionic acid | biospider | | L-α-alanine | Generator | | Propanoic acid, 2-amino-, (S) | biospider | | Propanoic acid, 2-amino-, (S)- | biospider |
|
|---|
| Chemical Formula | C3H7NO2 |
|---|
| IUPAC name | (2S)-2-aminopropanoic acid |
|---|
| InChI Identifier | InChI=1S/C3H7NO2/c1-2(4)3(5)6/h2H,4H2,1H3,(H,5,6)/t2-/m0/s1 |
|---|
| InChI Key | QNAYBMKLOCPYGJ-REOHCLBHSA-N |
|---|
| Isomeric SMILES | C[C@H](N)C(O)=O |
|---|
| Average Molecular Weight | 89.0932 |
|---|
| Monoisotopic Molecular Weight | 89.047678473 |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as alanine and derivatives. Alanine and derivatives are compounds containing alanine or a derivative thereof resulting from reaction of alanine at the amino group or the carboxy group, or from the replacement of any hydrogen of glycine by a heteroatom. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
| Direct Parent | Alanine and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Alanine or derivatives
- Alpha-amino acid
- L-alpha-amino acid
- Amino acid
- Carboxylic acid
- Monocarboxylic acid or derivatives
- Hydrocarbon derivative
- Organic oxygen compound
- Primary amine
- Organooxygen compound
- Organonitrogen compound
- Organic nitrogen compound
- Primary aliphatic amine
- Carbonyl group
- Amine
- Organopnictogen compound
- Organic oxide
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Physico-Chemical Properties - Experimental |
|---|
| Property | Value | Reference |
|---|
| Experimental logP | -2.85 | SANGSTER (1994) |
|---|
| Experimental Water Solubility | 164 mg/mL at 25 oC | YALKOWSKY,SH & DANNENFELSER,RM (1992) |
|---|
| Melting Point | Mp 297° dec. | DFC |
|---|
|
| Foods of Origin |
|---|
| Food | Content Range | Average | Reference |
|---|
| Food | | | Reference |
|---|
|
| Production Data |
|---|
| Production Method | commercial |
|---|
| Production Method Reference | Not Available |
|---|
| Production Method Reference File | Not Available |
|---|
| Quantity Available | Production upon request, up to 2 g |
|---|
| Delivery Time | Not Available |
|---|
| Storage Form | solid |
|---|
| Storage Conditions | -80°C |
|---|
| Stability | Not Available |
|---|
| Purity | Not Available |
|---|
| Spectra |
|---|
| Spectral Data Upon Request | Not Available |
|---|
| Provider Information |
|---|
|
| Commercial Vendors |
|---|
| AKSci | G138 |
|---|
| Glentham | GM7668 |
|---|
| Glentham | GM0361 |
|---|
| MetaSci | HMDB0000161 |
|---|
| Sigma-Aldrich | HMDB0000161 |
|---|
| Toronto Research Chemicals | A481500 |
|---|