| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation date | 2015-10-09 22:28:16 UTC |
|---|
| Update date | 2017-01-19 02:36:12 UTC |
|---|
| FoodComEx ID | PC000097 |
|---|
| FoodDB Record | FDB010558 |
|---|
| Chemical Information |
|---|
| Name | Phenylacetic acid |
|---|
| Description | Found in essential oils, e.g. neroli, rose oil, free and as estersand is also present in grapes, raspberry, strawberry, cherimoya, other fruits, cheddar cheese, Swiss cheese, wine, black tea, peated malt and other foodstuffs. Flavouring ingredient
Phenyl acetate (or phenylacetate) is a carboxylic acid ester that has been found in the biofluids of patients with nephritis and/or hepatitis as well as patients with phenylketonuria (PKU). Excess phenylalanine in the body can be disposed of through a transamination process leading to the production of phenylpyruvate. The phenylpyruvate can be further metabolized into a number of products. Decarboxylation of phenylpyruvate gives phenylacetate, while a reduction reaction gives phenyllactate. The phenylacetate can be further conjugated with glutamine to give phenylacetyl glutamine. All of these metabolites can be detected in serum and urine of PKU patients. Phenyl acetate is also produced endogenously as the metabolite of 2-Phenylethylamine, which is mainly metabolized by monoamine oxidase to form phenyl acetate. 2-phenylethylamine is an "endogenous amphetamine" which may modulate central adrenergic functions, and the urinary phenyl acetate levels have been postulated as a marker for depression. (PMID: 17978765, 476920, 6857245). Phenylacetate is also found in essential oils, e.g. neroli, rose oil, free and as esters' and in many fruits. As a result it is used as a perfumery and flavoring ingredient.; Phenylacetic acid (abr. PAA and synonyms are: ?-toluic acid, benzeneacetic acid, alpha tolylic acid, 2-phenylacetic acid) is an organic compound containing a phenyl functional group and an acetic acid functional group. It is a white solid with a disagreeable odor. Because it is used in the illicit production of phenylacetone (used in the manufacture of meth/amphetamines), it is subject to controls in the United States. |
|---|
| CAS Number | 103-82-2 |
|---|
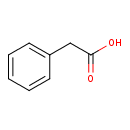
| Structure | |
|---|
| Synonyms | | Synonym | Source |
|---|
| 2-Phenylacetate | biospider | | 2-Phenylacetic acid | biospider | | 2-Phenylethanoate | biospider | | 2-Phenylethanoic acid | biospider | | a-Toluate | biospider | | a-Toluic acid | db_source | | alpha-Toluate | biospider | | alpha-Toluic acid | biospider | | alpha-Tolylic acid | biospider | | Benzenacetic acid | biospider | | Benzeneacetate | biospider | | Benzeneacetic acid | biospider | | Benzeneacetic acid, 9CI | db_source | | Benzylcarboxylic acid | biospider | | Benzylformate | Generator | | Benzylformic acid | biospider | | FEMA 2878 | db_source | | omega-Phenylacetate | biospider | | omega-Phenylacetic acid | biospider | | Phenyl-acetic acid | biospider | | Phenylacetate | manual | | Phenylethanoate | biospider | | Phenylethanoic acid | db_source | | Phenyllacetic acid | biospider | | W-Phenylacetate | HMDB | | W-Phenylacetic acid | HMDB | | α-toluate | Generator | | α-toluic acid | Generator |
|
|---|
| Chemical Formula | C8H8O2 |
|---|
| IUPAC name | 2-phenylacetic acid |
|---|
| InChI Identifier | InChI=1S/C8H8O2/c9-8(10)6-7-4-2-1-3-5-7/h1-5H,6H2,(H,9,10) |
|---|
| InChI Key | WLJVXDMOQOGPHL-UHFFFAOYSA-N |
|---|
| Isomeric SMILES | OC(=O)CC1=CC=CC=C1 |
|---|
| Average Molecular Weight | 136.1479 |
|---|
| Monoisotopic Molecular Weight | 136.0524295 |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as benzene and substituted derivatives. These are aromatic compounds containing one monocyclic ring system consisting of benzene. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Benzene and substituted derivatives |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Benzene and substituted derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Monocyclic benzene moiety
- Monocarboxylic acid or derivatives
- Carboxylic acid
- Carboxylic acid derivative
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Carbonyl group
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Physico-Chemical Properties - Experimental |
|---|
| Property | Value | Reference |
|---|
| Experimental logP | 1.41 | HANSCH,C ET AL. (1995) |
|---|
| Experimental Water Solubility | 16.6 mg/mL at 20 oC | CHIOU,CT et al. (1977) |
|---|
| Melting Point | Mp 77-78.5° | DFC |
|---|
|
| Foods of Origin |
|---|
| Food | Content Range | Average | Reference |
|---|
| Food | | | Reference |
|---|
|
| Production Data |
|---|
| Production Method | commercial |
|---|
| Production Method Reference | Not Available |
|---|
| Production Method Reference File | Not Available |
|---|
| Quantity Available | Production upon request, up to 500 mg |
|---|
| Delivery Time | Not Available |
|---|
| Storage Form | solid |
|---|
| Storage Conditions | -80°C |
|---|
| Stability | Not Available |
|---|
| Purity | Not Available |
|---|
| Spectra |
|---|
| Spectral Data Upon Request | Not Available |
|---|
| Provider Information |
|---|
|
| Commercial Vendors |
|---|
| Toronto Research Chemicals | P319180 |
|---|