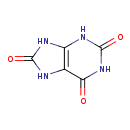
| Description | Uric acid, also known as urate or lithate, belongs to the class of organic compounds known as xanthines. These are purine derivatives with a ketone group conjugated at carbons 2 and 6 of the purine moiety. An oxopurine in which the purine ring is substituted by oxo groups at positions 2, 6, and 8. Uric acid is an extremely weak basic (essentially neutral) compound (based on its pKa). Uric acid exists in all living species, ranging from bacteria to humans. uric acid can be biosynthesized from xanthine through its interaction with the enzyme xanthine dehydrogenase/oxidase. In humans, uric acid is involved in the metabolic disorder called the purine nucleoside phosphorylase deficiency pathway. Outside of the human body, Uric acid is found, on average, in the highest concentration within milk (cow) and garden cress. Uric acid has also been detected, but not quantified in, several different foods, such as mamey sapotes, american pokeweeds, horned melons, towel gourds, and mammee apples. This could make uric acid a potential biomarker for the consumption of these foods. Uric acid is a potentially toxic compound. Uric acid, with regard to humans, has been found to be associated with several diseases such as bacterial meningitis, gout, and nucleotide depletion syndrome; uric acid has also been linked to several inborn metabolic disorders including primary hypomagnesemia and 3-methyl-crotonyl-glycinuria. |
|---|
| Synonyms | | Synonym | Source |
|---|
| 1H-Purine-2, 6,8-triol | biospider | | 1H-Purine-2,6,8-triol | biospider | | 1H-Purine-2,6,8-triol 2,6,8-Trihydroxypurine | biospider | | 1H-Purine-2,6,8(3H)-trione, 7,9-dihydro- | biospider | | 1H-Purine-2,6,8(3H)-trione, 7,9-dihydro- (9CI) | biospider | | 2,6-dihydroxy-7,9-dihydro-8H-purin-8-one | biospider | | 2,6, 8-Trioxypurine | biospider | | 2,6,8-Trihydroxypurine | db_source | | 2,6,8-Trioxopurine | biospider | | 2,6,8-Trioxypurine | biospider | | 2,6,8(1H,3H,9H)-Purinetrione | db_source | | 6,8-Dioxo-6,7,8,9-tetrahydro-1H-purin-2-olate | biospider | | 7,9-Dihydro-1H-purine-2,6,8(3H)-trione | biospider | | 7,9-Dihydro-1H-purine-2,6,8(3H)-trione, 9CI | db_source | | 7,9-dihydro-3H-purine-2,6,8-trione | biospider | | 7H-purine-2,6,8-triol | biospider | | 8-Hydroxyxanthine | biospider | | 9H-purine-2,6,8-triol | biospider | | Acid urate, ammonium | biospider | | Acid urate, sodium | biospider | | Acid, uric | biospider | | Acidum Uricum-Injeel Forte Liq (D6-D200) | biospider | | Ammonium acid urate | biospider | | Lithate | biospider | | Lithic acid | biospider | | Monohydrate, monosodium urate | biospider | | Monohydrate, sodium urate | biospider | | Monosodium urate | biospider | | Monosodium urate monohydrate | biospider | | Potassium urate | biospider | | purine-2,6,8-(1H,3H,9H)-trione | biospider | | Purine-2,6,8-triol | biospider | | Purine-2,6,8(1H,3H,9H)-trione | biospider | | Purine-3,6,8(1H,3H,9H)-trione | biospider | | Sodium acid urate | biospider | | Sodium acid urate monohydrate | biospider | | Sodium urate | biospider | | Sodium urate monohydrate | biospider | | Trioxopurine | biospider | | Urate | biospider | | Urate monohydrate, monosodium | biospider | | Urate monohydrate, sodium | biospider | | Urate, ammonium acid | biospider | | Urate, monosodium | biospider | | Urate, potassium | biospider | | Urate, sodium | biospider | | Urate, sodium acid | biospider | | URC | biospider | | Uric acid (8CI) | biospider | | Uric oxide | biospider | | Uricum acidum | biospider | | Uricum Acidum 3-30ch | biospider |
|
|---|