| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation date | 2015-10-09 22:27:32 UTC |
|---|
| Update date | 2017-01-19 02:36:10 UTC |
|---|
| FoodComEx ID | PC000030 |
|---|
| FoodDB Record | FDB012535 |
|---|
| Chemical Information |
|---|
| Name | L-Glutamic acid |
|---|
| Description | L-glutamic acid (abbreviated Glu or E), also referred to as glutamate (the anion), is a non-essential amino acid, one of the 20 amino acids used in the biosynthesis of proteins. L-glutamic acid exists in all living species, from bacteria to humans. In humans, dietary proteins are broken down by digestion into amino acids, which serves as metabolic fuel or other functional roles in the body. Glutamate is a component amino acid in many protein rich foods including the gluten protein in flour and is found as a free acid in cheeses and soya sauce. It is used as a flavor enhancer as a sodium salt known as monosodium glutamate. Glutamate is a key molecule in cellular metabolism. Glutamate is the most abundant fast excitatory neurotransmitter in the mammalian nervous system. At chemical synapses, glutamate is stored in vesicles. Nerve impulses trigger release of glutamate from the pre-synaptic cell. In the opposing post-synaptic cell, glutamate receptors, such as the N-methyl-d-aspartate acid (NMDA) receptor, bind glutamate and are activated. Because of its role in synaptic plasticity, it is believed that glutamic acid is involved in cognitive functions like learning and memory in the brain. Glutamate transporters are found in neuronal and glial membranes. They rapidly remove glutamate from the extracellular space. In brain injury or disease, glutamate can accumulate outside cells, causing glutamate excitotoxicity. Excitotoxicity occurs when neurons are exposed to high levels of glutamate or other neurotransmitters, causing persistent activation of the and α-amino-3-hydroxy-5-methylisoxazole propionic acid (AMPA) receptors and voltage-gated calcium channels. This results in a lethal influx of extracellular calcium leading to neuronal damage and eventual cell death. Cell death arises from damage to mitochondria by the excessively high intracellular calcium, which open mitochondrial pores, causing mitochondria to swell. Reactive oxygen species (ROS) may also be released by mitochondria into the intracellular space. Excess glutamate and calcium trigger apoptosis by further activating transcription factors for pro-apoptotic genes, or downregulating transcription factors for anti-apoptotic genes. Glutamate excitotoxicity causes other health consequences. In ischemic stroke and brain trauma, the severely reduced blood supply leads to flooding of glutamate and aspartate into the extra-neuronal space ( PMID: 16314180). Glutamate excitotoxicity is associated with diseases like amyotrophic lateral sclerosis, multiple sclerosis, lathyrism, and Alzheimer's disease (PMID: 20229265) and in epileptic seizures. Microinjection of glutamic acid into neurons produces spontaneous depolarization around one second apart which is like a paroxysmal depolarizing shift seen in epileptic attacks. This change in the resting membrane potential at seizure foci could cause spontaneous opening of voltage activated calcium channels, leading to glutamic acid release and further depolarization. Moreover, glutamic acid is associated with N-acetylglutamate synthetase deficiency, which is an inborn error of metabolism. |
|---|
| CAS Number | 56-86-0 |
|---|
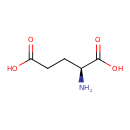
| Structure | |
|---|
| Synonyms | | Synonym | Source |
|---|
| (2S)-2-Aminopentanedioate | HMDB | | (2S)-2-Aminopentanedioic acid | HMDB | | (S)-(+)-Glutamate | HMDB | | (S)-(+)-Glutamic acid | HMDB | | (S)-2-Aminopentanedioate | Generator | | (S)-2-Aminopentanedioic acid | ChEBI | | (S)-Glutamate | Generator | | (S)-Glutamic acid | ChEBI | | α-aminoglutaric acid, (S)- | biospider | | α-glutamic acid, (S)- | biospider | | 1-Aminopropane-1,3-dicarboxylic acid | HMDB | | 2-Aminoglutaric acid, (S)- | biospider | | a-aminoglutaric acid, (S)- | biospider | | a-glutamic acid, (S)- | biospider | | Acide glutamique | ChEBI | | Acido glutamico | ChEBI | | Alpha-aminoglutarate, (S)- | biospider | | Alpha-aminoglutaric acid, (S)- | biospider | | Alpha-glutamate, (S)- | biospider | | Alpha-glutamic acid, (S)- | biospider | | E | ChEBI | | Glutamic acid; L-form | db_source | | Glutaminol | HMDB | | Glutaton | HMDB | | L (+)-glutamic acid, alpha-form | biospider | | L-(+)-Glutamate | HMDB | | L-(+)-Glutamic acid | HMDB | | L-a-Aminoglutarate | HMDB | | L-a-Aminoglutaric acid | HMDB | | L-alpha-Aminoglutarate | HMDB | | L-alpha-Aminoglutaric acid | HMDB | | L-Glu | ChEBI | | L-Glutamate | Generator | | L-glutamic acid | manual | | L-Glutaminic acid | ChEBI | | L-Glutaminsaeure | ChEBI | | S-1-Aminopropane-1,3-dicarboxylate | biospider |
|
|---|
| Chemical Formula | C5H9NO4 |
|---|
| IUPAC name | (2S)-2-aminopentanedioic acid |
|---|
| InChI Identifier | InChI=1S/C5H9NO4/c6-3(5(9)10)1-2-4(7)8/h3H,1-2,6H2,(H,7,8)(H,9,10)/t3-/m0/s1 |
|---|
| InChI Key | WHUUTDBJXJRKMK-VKHMYHEASA-N |
|---|
| Isomeric SMILES | N[C@@H](CCC(O)=O)C(O)=O |
|---|
| Average Molecular Weight | 147.1293 |
|---|
| Monoisotopic Molecular Weight | 147.053157781 |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as glutamic acid and derivatives. Glutamic acid and derivatives are compounds containing glutamic acid or a derivative thereof resulting from reaction of glutamic acid at the amino group or the carboxy group, or from the replacement of any hydrogen of glycine by a heteroatom. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
| Direct Parent | Glutamic acid and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Glutamic acid or derivatives
- Alpha-amino acid
- L-alpha-amino acid
- Amino fatty acid
- Dicarboxylic acid or derivatives
- Fatty acid
- Fatty acyl
- Amino acid
- Carboxylic acid
- Organic oxide
- Primary amine
- Organooxygen compound
- Organonitrogen compound
- Primary aliphatic amine
- Organopnictogen compound
- Carbonyl group
- Organic oxygen compound
- Amine
- Organic nitrogen compound
- Hydrocarbon derivative
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Physico-Chemical Properties - Experimental |
|---|
| Property | Value | Reference |
|---|
| Experimental logP | -3.69 | HANSCH,C ET AL. (1995) |
|---|
| Experimental Water Solubility | 8.57 mg/mL at 25 oC | BULL,HB et al. (1978) |
|---|
| Melting Point | Mp 224-225 (211-213°, 247-249°)° dec. | DFC |
|---|
|
| Foods of Origin |
|---|
| Food | Content Range | Average | Reference |
|---|
| Food | | | Reference |
|---|
|
| Production Data |
|---|
| Production Method | commercial |
|---|
| Production Method Reference | Not Available |
|---|
| Production Method Reference File | Not Available |
|---|
| Quantity Available | Production upon request, up to 2 g |
|---|
| Delivery Time | Not Available |
|---|
| Storage Form | solid |
|---|
| Storage Conditions | -80°C |
|---|
| Stability | Not Available |
|---|
| Purity | Not Available |
|---|
| Spectra |
|---|
| Spectral Data Upon Request | Not Available |
|---|
| Provider Information |
|---|
|
| Commercial Vendors |
|---|
| AKSci | C742 |
|---|
| Glentham | GM3277 |
|---|
| Glentham | GM8976 |
|---|
| MetaSci | HMDB0000148 |
|---|
| Sigma-Aldrich | HMDB0000148 |
|---|
| Toronto Research Chemicals | G596960 |
|---|