| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation date | 2015-10-09 22:27:11 UTC |
|---|
| Update date | 2017-01-19 02:36:09 UTC |
|---|
| FoodComEx ID | PC000001 |
|---|
| FoodDB Record | FDB009020 |
|---|
| Chemical Information |
|---|
| Name | Betaine |
|---|
| Description | Betaine, also known as Bet or trimethylglycine, belongs to the class of organic compounds known as alpha amino acids. These are amino acids in which the amino group is attached to the carbon atom immediately adjacent to the carboxylate group (alpha carbon). Betaine is a moderately acidic compound (based on its pKa). Betaine exists in all eukaryotes, ranging from yeast to humans. In humans, betaine is involved in betaine metabolism. Betaine is a bland tasting compound. Outside of the human body, Betaine is found, on average, in the highest concentration within a few different foods, such as swamp cabbages, quinoa, and lambsquarters and in a lower concentration in common buckwheats, burdocks, and yellow zucchinis. Betaine has also been detected, but not quantified in, several different foods, such as lemon verbena, wasabis, endives, chickpea, and pomegranates. This could make betaine a potential biomarker for the consumption of these foods. Intracellular accumulation of betaines permits water retention in cells, thus protecting from the effects of dehydration (Wikipedia). Betaine comes from either the diet or by the oxidation of choline. Betaine, with regard to humans, has been found to be associated with several diseases such as chronic renal failure, colorectal cancer, and alzheimer's disease; betaine has also been linked to several inborn metabolic disorders including argininosuccinic aciduria and propionic acidemia. In an acute toxicology study in rats, death frequently occurred at doses equal to or greater than 10,000 mg/kg. Betaine has been shown to have an inhibitory effect on NO release in activated microglial cells and may be an effective therapeutic component to control neurological disorders (PMID: 22801281). Betaine insufficiency is associated with metabolic syndrome, lipid disorders, and diabetes, and may have a role in vascular and other diseases (PMID: 20346934). Many reports have shown that betaine's therapeutic effectiveness is limited, and does not lower tHcy levels or prevent clinical symptoms . |
|---|
| CAS Number | 107-43-7 |
|---|
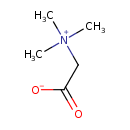
| Structure | |
|---|
| Synonyms | | Synonym | Source |
|---|
| (carboxymethyl)trimethylammonium hydroxide inner salt | biospider | | (carboxymethyl)trimethylammonium hydroxide, inner salt | biospider | | (carboxymethyl)trimethylammonium inner salt | biospider | | (trimethyl-α-earleine | biospider | | (trimethylammonio)acetate | biospider | | (trimethylammoniumyl)acetate | biospider | | (Trimethylammoniumyl)acetic acid | Generator | | α-earleine | biospider | | βine | biospider | | βine (van) | biospider | | 1-carboxy-N,N,N-trimethyl-Methanaminium | biospider | | 1-carboxy-N,N,N-trimethyl-Methanaminium hydroxide | biospider | | 1-Carboxy-N,N,N-trimethylmethanaminium inner salt | biospider | | 2-(Trimethylammonio)ethanoic acid, hydroxide, inner salt | biospider | | 2-N,N,N-Trimethylammonio acetate | ChEBI | | 2-N,N,N-Trimethylammonio acetic acid | Generator | | A-earleine | biospider | | Abromine | biospider | | Acidin-pepsin | biospider | | Acidol | ChEBI | | Alpha-earleine | biospider | | Aminocoat | biospider | | Aquadew AN 100 | biospider | | Bet | ChEBI | | Betafin | biospider | | Betafin BCR | biospider | | Betafin BP | biospider | | Betaine | biospider | | Betaine (8CI) | biospider | | Betaine hydrochloride | biospider | | Betaine, anhydrous | biospider | | Betainum muriaticum | biospider | | Cystadane | biospider | | Cystadane (TN) | biospider | | Ektasolve ee | HMDB | | Finnstim | biospider | | Glycine βine | biospider | | Glycine betaine | biospider | | Glycine, trimethylβine | biospider | | Glycine, trimethylbetaine | biospider | | Glycocoll βine | biospider | | Glycocoll betaine | biospider | | GLYCYLβine | biospider | | Glycylbetaine | biospider | | Glykokollbetain | biospider | | Greenstim | biospider | | Jortaine | biospider | | Loramine amb 13 | HMDB | | Loramine AMB-13 | biospider | | Lycine | biospider | | Methanaminium, 1-carboxy-N,N,N-trimethyl-, hydroxide | biospider | | Methanaminium, 1-carboxy-N,N,N-trimethyl-, inner salt | biospider | | Methanaminium, 1-carboxy-N,N,N-trimethyl-, inner salt (9CI) | biospider | | Methanaminium, carboxy-n,n,n-trimethyl-, inner salt | biospider | | N,n,n-trimethylammonioacetate | biospider | | N,N,N-Trimethylammonioacetic acid | Generator | | N,n,n-trimethylglycine | biospider | | Oxyneurine | biospider | | Rubrine c | biospider | | Trimethylaminoacetate | biospider | | Trimethylaminoacetic acid | biospider | | Trimethylammonioacetate | biospider | | Trimethylammonioacetic acid | Generator | | Trimethylbetaine glycine | biospider | | Trimethylglycine | biospider | | Trimethylglycocoll | biospider | | Trimethylglycocoll anhydride | biospider |
|
|---|
| Chemical Formula | C5H11NO2 |
|---|
| IUPAC name | 2-(trimethylazaniumyl)acetate |
|---|
| InChI Identifier | InChI=1S/C5H11NO2/c1-6(2,3)4-5(7)8/h4H2,1-3H3 |
|---|
| InChI Key | KWIUHFFTVRNATP-UHFFFAOYSA-N |
|---|
| Isomeric SMILES | C[N+](C)(C)CC([O-])=O |
|---|
| Average Molecular Weight | 117.1463 |
|---|
| Monoisotopic Molecular Weight | 117.078978601 |
|---|
| Chemical Taxonomy |
|---|
| Description | Belongs to the class of organic compounds known as alpha amino acids. These are amino acids in which the amino group is attached to the carbon atom immediately adjacent to the carboxylate group (alpha carbon). |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
| Direct Parent | Alpha amino acids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Alpha-amino acid
- Quaternary ammonium salt
- Tetraalkylammonium salt
- Carboxylic acid salt
- Monocarboxylic acid or derivatives
- Carboxylic acid
- Organopnictogen compound
- Hydrocarbon derivative
- Organic salt
- Organic oxygen compound
- Organooxygen compound
- Organonitrogen compound
- Carbonyl group
- Amine
- Organic nitrogen compound
- Organic oxide
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Physico-Chemical Properties - Experimental |
|---|
| Property | Value | Reference |
|---|
| Experimental logP | Not Available | |
|---|
| Experimental Water Solubility | 611 mg/mL at 19 oC | YALKOWSKY,SH & DANNENFELSER,RM (1992) |
|---|
| Melting Point | 293 oC ( Soicke, H., Fitoterapia 1988, V59(1), P73-5) | |
|---|
|
| Foods of Origin |
|---|
| Food | Content Range | Average | Reference |
|---|
| Food | | | Reference |
|---|
|
| Production Data |
|---|
| Production Method | commercial |
|---|
| Production Method Reference | Not Available |
|---|
| Production Method Reference File | Not Available |
|---|
| Quantity Available | Production upon request, up to 5 g |
|---|
| Delivery Time | Not Available |
|---|
| Storage Form | solid |
|---|
| Storage Conditions | -80°C |
|---|
| Stability | Not Available |
|---|
| Purity | Not Available |
|---|
| Spectra |
|---|
| Spectral Data Upon Request | Not Available |
|---|
| Provider Information |
|---|
|
| Commercial Vendors |
|---|
| AKSci | G671 |
|---|
| Glentham | GE1604 |
|---|
| MetaSci | HMDB0000043 |
|---|
| Sigma-Aldrich | HMDB0000043 |
|---|
| Toronto Research Chemicals | B325005 |
|---|